Abstract
Historically, patients with metastatic, persistent or recurrent cervical cancer had limited therapeutic options. Despite several Phase II/III clinical trials, the combination of cisplatin and paclitaxel remained the most effective chemotherapeutic regimen. In 2014, publication of Gynecologic Oncology Group 240 represented the emergence of an alternate and effective therapeutic option. This prospective, randomized, Phase III clinical trial explored the impact of adding the antiangiogenic agent bevacizumab to two separate cytotoxic chemotherapy backbones. Importantly, the study met its primary end point, showing a survival advantage of approximately 4 months without detriment in quality of life. As such, a review of bevacizumab and its application in patients with advanced-stage cervical cancer is warranted.
Keywords: bevacizumab, cervical cancer, pharmacodynamics, pharmacokinetics, prognostic criteria, quality of life, survival
The burden of gynecologic malignancies remains a stimulus toward scientific investigation and the discovery/development of novel therapeutic agents. In 2014, it is estimated that there will be 86,970 new cases of ovarian, uterine and cervical cancer in the USA, with 26,880 deaths [1]. Due to lack of an effective screening strategy, patients with ovarian cancer are diagnosed with an advanced stage disease and require surgical cytoreduction as well as systemic chemotherapy. Conversely, the Pap smear, an effective screening strategy for cervical cancer, has translated into prevention and early detection with improved survival. Globally, however, cervical cancer continues to be the most lethal gynecologic malignancy, with 529,800 new cases and 275,100 deaths in 2011 [2]. This discrepancy between global and regional disease burden is attributable to the disproportionately high number of cervical cancer cases in resource-poor countries that lack adequate infrastructure and screening programs.
Importantly, despite appropriate screening and early detection, a subset of patients with cervical cancer will present with metastatic disease or develop disease recurrence after primary therapy. In the context of metastatic or recurrent disease, a complete cure is rare, and treatment focuses on palliation of symptoms, disease control and prolongation of life [3]. Chemotherapeutic options for patients with advanced stage or recurrent cervical cancer have been explored and are based on clinical trials completed under the auspices of cooperative groups, most notably the Gynecologic Oncology Group (GOG). Since Thigpen’s initial paper in 1981, a number of single drug and combination regimens have been studied in the treatment of advanced and metastatic cervical cancer with limited gains in overall survival (OS) [4–20]. Ultimately, cisplatin + paclitaxel was established as the backbone for future trials, with OS approaching 13 months [4].
The poor oncologic outcome in this patient population catalyzed the exploration of novel treatment paradigms. In an era of personalized and molecular medicine, the development of biologic therapies, to be used alone or in conjunction with cytotoxic chemotherapy, is a clinical priority. The biologic agent with the greatest clinical experience in the gynecologic cancer arena is the antiangiogenic agent bevacizumab. With publication of GOG 240, bevacizumab was shown, for the first time, to improve both OS and progression-free survival without a significant decrement in quality of life (QoL) in a patient population previously lacking effective therapeutic options (i.e., women with advanced cervical cancer). This trial led to regulatory approval on 14 August 2014 by the US FDA for bevacizumab in this population [21].
This review article will discuss the pharmacokinetics/pharmacodynamics of bevacizumab, its clinical efficacy in the treatment of patients with advanced stage, persistent or recurrent cervical cancer, as well as QoL implications, biomarker discovery, and potential predictors of response.
Bevacizumab in solid malignancies
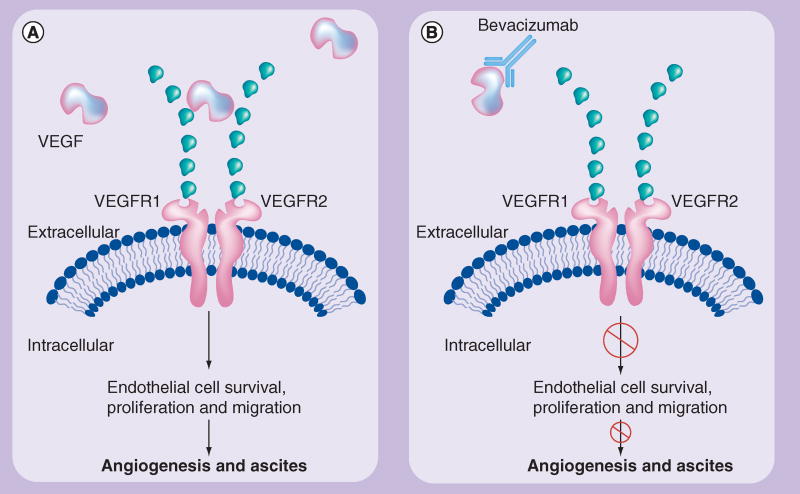
Bevacizumab is a recombinant humanized monoclonal antibody that selectively binds to and neutralizes the biologic activity of VEGF (Figure 1) [22]. The drug is produced by using recombinant DNA technology in a Chinese hamster ovarian cell expression system, in a nutrient medium containing the antibiotic gentamicin which is purified by a process that includes viral inactivation and removal [23].
Figure 1.
Bevacizumab mode of action: binding and neutralizing VEGF ligand, preventing interaction with the transmembrane receptor.
Adapted with permission from [22].
Bevacizumab was first studied in patients with renal cell carcinoma, because of its unique VEGF-driven biology, and five other common solid tumors with high therapeutic need: colorectal, prostate, lung and breast cancers, and glioblastoma [24]. Additional studies were conducted in patients with wet age-related macular degeneration, showing results comparable to the previously used ranibizumab [25]. Phase III bevacizumab trials were then conducted in metastatic colorectal cancer [26,27], metastatic non-small-cell lung cancer [28], metastatic breast cancer (mBC) [29] and recurrent glioblastoma [30,31], all of which met their primary end points, thus supporting FDA approval of bevacizumab for these indications (Table 1) [32]. Importantly, the accelerated approval of bevacizumab in patients with mBC was reversed by the FDA in 2011, after prolonged follow-up failed to show an OS improvement. Analogously, despite four prospective Phase III clinical trials illustrating an improved progression-free survival (PFS) in patients with ovarian cancer, lack of an OS advantage in the bevacizumab containing arms has been an impediment to FDA approval in this disease (Table 2) [33–39].
Table 1.
Registration trials resulting in US FDA approval of bevacizumab.
| Study | Disease site | n | Eligibility criteria |
Regimen studied | Outcome measure
|
AEs | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
|
Visual acuity |
Median PFS (months) |
Median OS (months) |
|||||||
|
Ocular indication
| |||||||||
| Martin et al. | AMD | 1208 | Visual acuity between 20/25 and 20/320 | Ranibizumab monthly | 8.0 letters gained | Equivalent rates of MI, death and stroke between arms | [25] | ||
| Bevacizumab monthly | 8.5 letters gained | ||||||||
|
| |||||||||
|
Solid tumors
| |||||||||
| Hurwitz et al. | Colorectal cancer | 813 | ECOG PS 0–1 | ILF + placebo | 6.2 | 15. 6 | Leukopenia (37%); diarrhea (32%); HTN (11%); bleeding (3%); GI perforation (1.5%) | [26] | |
| First line | ILF + bev 5 mg/kg iv. Q2w | 10.6 | 20.3 | ||||||
| Metastatic | (HR: 0.54; 95% CI: 0.45–0.66) | (HR: 0.66; 95% CI: 0.54–0.81) | |||||||
|
| |||||||||
| Giantonio et al. | Colorectal cancer | 829 | Adv stage | FOLFOX-4 + placebo | 4.7 | 10.8 | HTN (6%); emesis (10%); bleeding (3%); neuropathy (16%); thromboembolism (3%) | [27] | |
| Metastatic | FOLFOX-4 + bev 10 mg/kg Q2w | 7.3 | 13.0 | ||||||
| Bev 10 mg/kg Q2w | 2.7 | N/R | |||||||
| (HR: 0.52; 95% CI: 0.42–0.65)† | (HR: 0.75; 95% CI: 0.63–0.89) | ||||||||
|
| |||||||||
| Sandler et al. | Nonsquamous NSCLC | 878 | ECOG PS 0–1 | Carbo/paclitaxel+ placebo | 4.8 | 10.3 | Leukopenia (25%); HTN (7%); proteinuria (3%); bleeding (4%) | [28] | |
| First line | Carbo/paclitaxel + bev 15 mg/kg | 6.4 | 12.3 | ||||||
| Locally adv Metastatic Recurrent | Q3w | (HR: 0.65; 95% CI: 0.56–0.76) | (HR: 0.80; 95% CI: 0.69–0.93) | ||||||
|
| |||||||||
| Miller et al. | Breast cancer | 722 | ECOG 0−1 | Paclitaxel + placebo | 5.8 | 24.8 | Infection (9%); Fatigue (9%); HTN (15%); neuropathy (23%) | [29] | |
| Locally recurrent Metastatic | Paclitaxel + bev 10 mg/kg Q2w | 11.4 (HR: 0.42; 95% CI: 0.34–0.52) | 26.5 (HR: 0.87; 95% CI: 0.72–1.05)‡ | ||||||
|
| |||||||||
| Friedman et al. | GBM | 167 | KPS >70% | Bev 10 mg/kg Q2w | 42.6% (6 months PFS) | 9.3 | HTN (8%); thromboembolic disease (6%) | [30] | |
| First or second recurrence | Bev 10 mg/kg Q2w + irinotecan | 50.3% (6 months PFS) | 8.8 | ||||||
|
| |||||||||
| Kreisl et al. | GBM | 48 | KPS >60% | Bev 10 mg/kg Q2w | 16 weeks | 31 weeks | Thromboembolism (12.5%); HTN (4%); hypophosphatemia (4%) | [31] | |
| Recurrent (no limit on number of prior therapies) | Transition to bev + irinotecan at progression | 29% (6 months PFS) | 57% (6 months survival) | ||||||
| Arm closed early due to futility | |||||||||
|
| |||||||||
| Escudier et al. | Renal cell cancer | 649 | KPS >70% | IFN-α-2a + placebo | 5.4§ | 21.3 | Fatigue (12%); asthenia (10%); proteinuria (7%); HTN (3%); bleeding (3%) | [32] | |
| First line | IFN-α-2a + bev 10 mg/kg Q2w | 10.2§ | 23.3 | ||||||
| Metastatic No CNS mets | (HR: 0.63; 95% CI: 0.52–0.75) | (HR: 0.91; 95% CI: 0.76 –1.10) | |||||||
|
| |||||||||
| AURELIA | Ovarian cancer | 361 | ECOG PS 0–2 | iv. paclitaxel or iv. topotecan or iv. PLD | 3.4 | 13.3 | HTN (20.1%); proteinuria (12.8%); fatigue (2.2%); GI perforation (1.7%); thromboembolic disease (3.4%) | [39]¶ | |
| Platinum-resistant recurrence | Chemo as above + bev 15 mg/kg Q3w | 6.7 | 16.6 | ||||||
| (HR: 0.48; 95% CI: 0.36–0.60) | (HR: 0.85; 95% CI: 0.66 –1.08) | ||||||||
|
| |||||||||
| Tewari et al. | Cervical cancer | 452 | GOG PS 0–1 | Chemotherapy + placebo | 5.9 | 13.3 | Fistula (3%); HTN (2%); neutropenia (35%); thromboembolism (8%); bleeding (5%) | [21]¶ | |
| Recurrent/ persistent or metastatic | Chemotherapy + bev 15 mg/kg Q3w | 8.2 | 17.0 | ||||||
| (HR: 0.67; 95% CI: 0.54–0.82) | (HR: 0.71; 97% CI: 0.54–0.94) | ||||||||
Diference between FOLFOX + placebo vs FOLFOX + bev.
Lack of a signifcant OS advantage resulted in the US FDA revoking initial approval of bevacizumab use in patients with metastatic/recurrent breast cancer.
Signifcantly greater than historical controls for both treatment arms (p < 0.0001).
Regulatory approval granted by the US FDA on 14 August 2014 (recurrent/persistent and metastatic cervical cancer) and on 14 November 2014 (platinum-resistant ovarian cancer).
Adv: Advanced; AE: Grade 3 or 4 adverse events on bevacizumab; AMD: Age-related macular degeneration; Bev: Bevacizumab; Carbo: Carboplatin; Chemo: Paclitaxel + cisplatin vs paclitaxel + topotecan; ECOG: Eastern Cooperative Oncology Group; FOLFOX-4: Oxaliplatin + leucovorin + fuorouracil; GBM: Glioblastoma; GI: Gastrointestinal; GOG: Gynecologic Oncology Group; HR: Hazard ratio; HTN: Hypertension; ILF: Irinotecan + fuorouracil + leucovorin; iv.: Intravenous; KPS: Karnofsky performance score; Mets: Metastases; MI: Myocardial infarction; NSCLC: Non-small-cell lung cancer; OS: Overall survival; PFS: Progression-free survival; PLD: Pegylated liposomal doxorubicin; PS: Performance status; Q2w: Every 2 weeks; Q3w: Every 3 weeks.
Table 2.
Prospective Phase III clinical trials incorporating bevacizumab in the treatment of ovarian cancer indicating a progression-free survival advantage, but no overall survival advantage.
| Trial | n | Eligibility | Arms | Grade 3–4 AEs† | Primary end point |
Secondary end point | Ref. |
|---|---|---|---|---|---|---|---|
| GOG 218 | 1873 | Incompletely and completely‡ resected stage 3 or any stage 4; GOG PS 0–2 | iv. carboplatin (AUC 5) + iv. paclitaxel (175 mg/m2) + placebo followed by maintenance placebo Q3w iv. carboplatin (AUC 5) + iv. paclitaxel (175 mg/m2) + iv. bevacizumab (15 mg/kg) + placebo maintenance Q3w iv. carboplatin (AUC 5) + iv. Paclitaxel (175 mg/m2) + iv. bevacizumab (15 mg/kg) + iv. bevacizumab (15 mg/kg) maintenance Q3w | HTN; (22.9%); GI events (2.6%); proteinuria (1.6%); VTE (6.7%) | Median PFS; 10.3 vs 11.2 vs 14.1 months; HR: 0.717; (0.625–0.824); p < 0.001 | Median OS; 39.3 vs 38.7 vs 39.7 months; HR: 0.915 (0.727–1.15); p = 0.45 | [35] |
| ICON 7 | 1528 | Stage 1–2A (clear cell, grade 3); stage 2B-4; ECOG PS 0–2 | iv. carboplatin (AUC 5) + iv. paclitaxel (175 mg/m2) Q3w iv. carboplatin (AUC 5) + iv. paclitaxel (175 mg/m2) + iv. bevacizumab (7.5mg/kg) + iv. bevacizumab (7.5 mg/kg) maintenance Q3w | Bleeding (1%); HTN (6%); VTE (4%); GIP (1%); neutropenia (17%) | Median PFS; 17.3 vs 19.0 months; HR: 0.81 (0.70–0.94); p = 0.0041 | Median OS; 58.6 vs 58 months; HR: 0.99 (0.85–1.14); p = 0.85 | [34] |
| OCEANS | 484 | Platinum sensitive recurrent ovarian cancer§; ECOG PS 0–1 | iv. carboplatin (AUC 4) + iv. gemcitabine (1000 mg/m2) + placebo Q3w iv. carboplatin (AUC 4) + iv. gemcitabine (1000 mg/m2) + iv. bevacizumab (15 mg/kg) Q3w | HTN (17.4%); proteinuria (8.5%); bleeding (5.7%); F/A (1.6%); VTE (4%) | Median PFS; 8.4 vs 12.4 months; HR: 0.484 (0.388–0.605); p < 0.0001 | OS data immature; ORR; 78.5 vs 57.4%; p < 0.0001, DOR; 10.4 vs 7.4 months; HR: 0.534 (0.408–0.698) | [36] |
| AURELIA# | 361 | Platinum resistant recurrence¶; ≤2 prior chemotherapy regimens; no e/o rectosigmoid involvement; ECOG PS 0–2 | iv. paclitaxel (80 mg/m2) days 1, 8, 15, 22 Q4w or iv. topotecan (4 mg/m2) days 1, 8, 15 Q4w or iv. PLD (40 mg/m2) Q4w Chemotherapy as above plus iv. bevacizumab (15 mg/kg) Q3w | HTN (20.1%); proteinuria (12.8%); F/A (2.2%); GIP (1.7%); VTE (3.4%) | Median PFS; 3.4 vs 6.7 months; HR: 0.48; (0.36–0.60); p < 0.001 | Median OS; 13.3 vs 16.6 months; HR: 0.85 (0.66–1.08); p = 0.174 | [39] |
GOG 213 is a randomized Phase III trial designed to determine whether secondary cytoreductive surgery and/or the incorporation of bevacizumab to second-line chemotherapy improves progression-free survival in patients with platinum-sensitive recurrent ovarian cancer. At the time of manuscript production the data from GOG 213 were under embargo by the Society of Gynecologic Oncology.
†Investigational arms; a: Maintenance vs chemotherapy only arm; b: control vs bevacizumab throughout arms.
Investigational arms; a: Maintenance vs chemotherapy only arm; b: control vs bevacizumab throughout arms.
After protocol modifcation patients with optimally resected stage 3 disease were eligible.
Progression-free interval at least 6 months.
Based on the data from the AURELIA study, the US FDA approved bevacizumab for platinum-resistant, recurrent epithelial ovarian cancer on 14 November 2014 (see Table 1)
Progression-free interval less than or equal to 6 months.
AE: Adverse events; DOR: Duration of response; e/o: Evidence of; ECOG: Eastern Cooperative Oncology Group; F/A: Fistula/abscess; GIP: Gastrointestinal perforation; HR: Hazard ratio; HTN: Hypertension; iv.: Intravenous; ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival; PLD: Pegylated liposomal doxorubicin; PS: Performance status; VTE: Venous thromboembolism; Q3w: Every 3 weeks; Q4w: Every 4 weeks. Adapted with permission from [37].
Conversely, when studied in patients with advanced stage, recurrent or persistent cervical cancer in GOG 240, bevacizumab was associated with a significant improvement in PFS and OS, without a decrement in measured QoL parameters [21]. The above represents the first time a targeted agent resulted in an OS advantage in patients with gynecologic cancer, with practice changing implications.
The combination of a significant improvement in PFS with a loss of significance at the time of OS analysis has resulted in continued debate regarding appropriate study end points. In diseases where additional effective therapies are available at the time of progression (on study), a meaningful PFS difference is commonly diluted by postprogression treatment and crossover. This is best represented by recent data indicating that over 50% of subjects with advanced ovarian cancer treated on an antiangiogenic trial received seven or more cytotoxic anticancer regimens [36]. The above paradigm likely explains the lack of a significant OS difference with the use of bevacizumab in patients with recurrent or advanced stage breast and ovarian cancers, where cytotoxic chemotherapy regimens are effective at prolonging life after disease progression.
Conversely, in the setting of advanced stage or recurrent cervical cancer, no effective therapies exist, and the survival advantage of bevacizumab is maintained [40]. As explained by Broglio et al., a significant PFS advantage (hazard ration [HR]: 0.73; p = 0.03) translates into a significant OS advantage (HR 0.61; p = 0.008) when median survival postprogression on study is estimated to be 6 months [40]. Conversely, in the setting where alternate treatment options exist, the postprogression survival is extended, and the survival advantage is diluted. With an analogous calculated PFS, in a simulated study, the HR for OS would rise to 1.29 (p = 0.262) if postprogression survival is estimated to be 18 months.
Antiangiogenic therapy in cervical cancer: the biologic rational
Patients with cervical cancer are routinely exposed to radiation and chemotherapy at the time of primary therapy, potentially altering disease biology. Chemosensitizing radiation may select for radio-resistant and chemotherapy-resistant cell populations, particularly if there is crossover with respect to mechanisms of drug resistance. Additionally, cancer foci recurring or persisting in the previously irradiated field may have compromised blood supply and associated relative hypoxia, limiting delivery of cytotoxic drugs. These unique characteristics may explain the limited response to retreatment with traditional chemotherapy in patients with recurrent cervical cancer, highlighting the importance of studying novel biologic strategies.
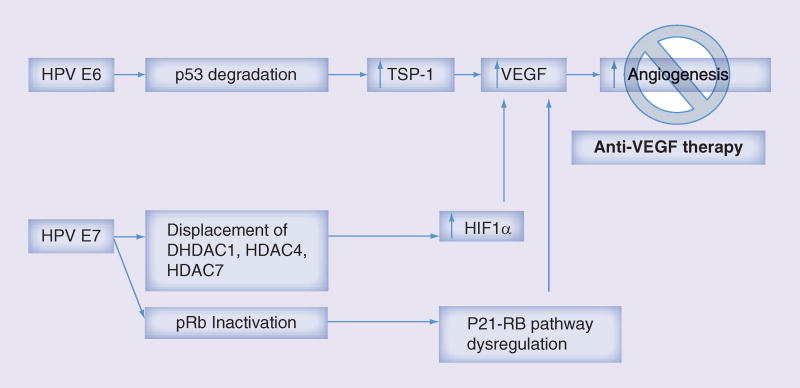
Biologically, abnormal vascularity identified on colposcopic examination may suggest invasive disease. Mechanistically, this is explained by the effects of E6/E7 on the angiogenic pathway. Upregulation of the E6 oncoprotein is hypothesized to directly stimulate VEGF production [41,42]. In transgenic mice experiments, investigators were able to reproduce invasive squamous cell carcinoma of the epidermis with E6 and E7 oncogene expression [43]. Ultimately, E6-mediated degradation of p53 and E7 inactivation of pRb result in increased VEGF and hypoxia inducible factor 1α, promoting angiogenesis and tumor growth (Figure 2).
Figure 2.
Biologic rational of bevacizumab use in cervical carcinoma.
Adapted with permission from [44].
Clinical evidence that angiogenesis plays a role in cervical cancer has accumulated over the last decade. VEGF-induced tumor angiogenesis has been associated with adverse oncologic outcomes in patients with cervical cancer [24,45–49]. In an early study, high intratumoral microvessel density was associated with poor prognosis and remained significant in a multivariable model [47]. More recently, intratumoral VEGF was shown to be upregulated in cervical cancer specimens relative to control cervical tissues, with higher VEGF levels being associated with advanced stage, increase risk of nodal metastasis, and worse PFS and OS [50–53]. Additionally, cluster of differentiation 31 expression, found on endothelial cell surfaces and used as an immunohistochemical marker of angiogenesis, has been shown to be significantly associated with tumor size and the presence of lymph vascular space involvement in patients with clinical stage 1B squamous cell cervical cancer [54].
Bevacizumab: pharmacokinetics & pharmacodynamics
Monoclonal antibodies have rapidly evolved into a robust segment of developmental therapeutics in the treatment of solid malignancies. There are several antibody isotypes (IgA, IgD, IgE, IgG and IgM) each with well-described pharmacologic properties. The most prevalent isotype, IgG, constitutes nearly 85% of serum immunoglobulins and is the primary derivative for therapeutic development secondary to its role in humoral protection [55].
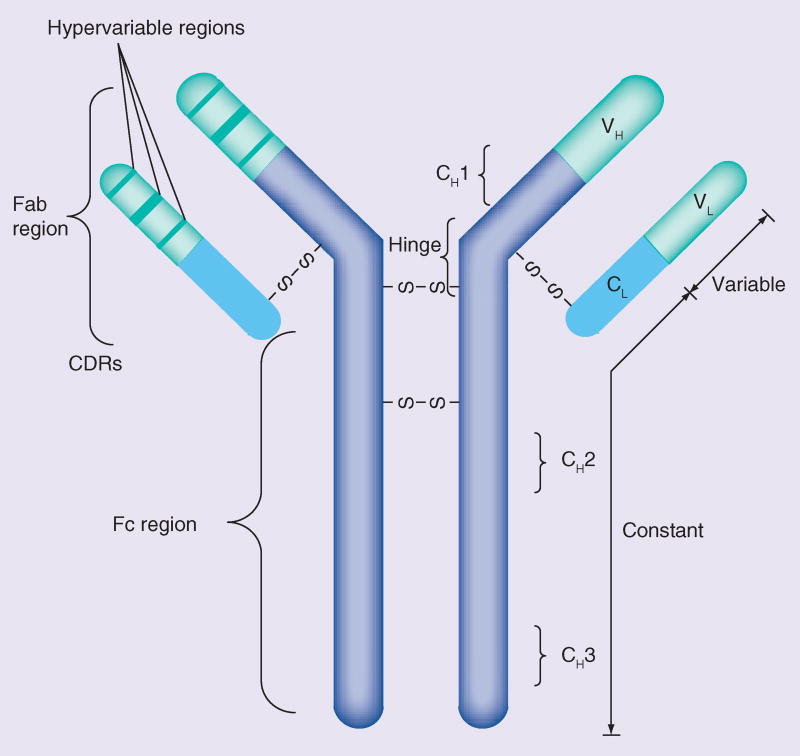
IgG monomers are constructed of four polypeptide chains: two heavy chains and two light chains connected by disulfide bonds at the ‘hinge region’ (Figure 3) [55]. The variable region contains short peptide sequences known as the complimentary determining regions, representing the antigen-binding site. The FC region consists of constant heavy chains involved in essential interactions with components of the immune system.
Figure 3.
Generalized structure of a monoclonal antibody monomer.
CDR: Complimentary determining region; CH: Constant heavy chain; CL: Constant light chain; S: Sulfde; VH: Variable heavy chain; VL: Variable light chain.
Adapted with permission from [55].
The pharmacokinetics of bevacizumab was initially described using a two-compartment model. Bevacizumab deposition is characterized by low clearance and a long elimination half-life. These characteristics allow for predictable target tissue levels despite variable dosing schedules (ranging from every 2–3 weeks on clinical trials) [56].
In population-based studies, there was no identifiable difference in bevacizumab pharmacokinetics in relation to age. Conversely, hypoalbuminemia and high tumor burden resulted in more rapid drug clearance (19% faster in patients with low levels of serum albumin [<29 g/l] and 7% faster in subjects with higher tumor burden) [56].
In clinical trials, the typical value for central volume (Vc) was 2.73 l for female patients, with a peripheral volume (Vp) of 1.69 l [23].
Furthermore, the evaluation of bevacizumab metabolism in rabbits mirrored that expected for a native IgG molecule which does not bind to VEGF [23]. This metabolism was predominantly mediated by proteolytic catabolism and is independent of renal or hepatic elimination. The binding of IgG to the FcRn receptor results in protection from cellular metabolism and a long terminal half-life [57,58]. Importantly, the elimination pharmacokinetics of bevacizumab is linear at doses ranging from 1.5 to 10 mg/kg/week.
According to the two-compartmental model, the elimination half-life of bevacizumab is 18 days for a typical female patient [23,56].
Bevacizumab in cervical cancer
In the first case series describing the use of bevacizumab in patients with recurrent cervical cancer, despite heavy pretreatment (median of three prior regimens), there was a 67% overall response rate [59]. Treatment was well tolerated, with only one grade 4 adverse event (AE) noted (Table 3).
Table 3.
Phase II clinical trials of bevacizumab in cervical cancer.
| Study | Drug | n | Eligibility | Pathology | OS (months) |
PFS (months) |
RR (%) |
Grade 3–4 AEs | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Monk et al. | Bevacizumab 15 mg/kg Q3w | 46 | Second line (74%); third line (26%); GOG PS 0–2 | Squamous, adenosquamous | 7.3 | 3.4 | 35 | HTN (15%); thromboembolism (11%); anemia (4%); vaginal bleeding (2%); neutropenia (2%); pain (13%); GI (8.7%); cardiovascular (4.3%); pulmonary (2%); fistula (2%) | [60] |
| Schefter et al. | Cisplatin 40 mg/m2+ radiation therapy + brachytherapy + bevacizumab 10 mg/kg Q2w for three cycles | 49 | Untreated patients with st age 1B-3B cervical cancer | Squamous (80%) | NR | NR | NR | No treatment related SAEs; hematalogic AE 80% | [58] |
| Zighelboim et al. | Cisplatin 50 mg/m2 day 1 + topotecan 0.75 mg/m2 days 1, 2, 3 + bevacizumab 15 mg/kg day 1 Q3w | 27 | First recurrence; GOG PS 0–1 | Squamous (67%), adenocarcinoma (33%) | 13.2 | 7.1 | 35 | Leukopenia (74%); neutropenia (56%); thrombocytopenia (81%); anemia (63%); GI (19%); pain (33%); metabolic (48%); infection (19%) | [61] |
AE: Adverse event; GI: gastrointestinal; GOG: Gynecologic Oncology Group; HTN: Hypertension; NR: Not reported; OS: Overall survival; PFS: Progression-free survival; PS: Performance status; Q3w: Every 3 weeks; RR: Response rate; SAE: Serious adverse event.
These preliminary results catalyzed the development of GOG protocol 227C, a Phase II trial designed to evaluate the efficacy and tolerability of bevacizumab in the treatment of recurrent cervical cancer (Table 3) [61]. Among the 46 eligible and evaluable patients, 38 (82.6%) received prior pelvic radiation as well as either one (n = 34; 74%) or two (n = 12; 26%) cytotoxic regimens for recurrent disease. Eleven patients (23.9%; two-sided 90% CI: 14–37%) survived progression free for at least 6 months, and five patients (10.9%; two-sided 90% CI: 4–22%) had partial responses, with a median response duration of 6.2 months (range, 2.83–8.28 months). The median PFS and OS times were 3.40 months (95% CI: 2.53–4.53 months) and 7.29 months (95% CI: 6.11–10.41 months), respectively. These results compared favorably with historical Phase II trials in this setting [62].
Given the clinical activity noted in the pretreated population, Radiation Therapy Oncology Group protocol 0417 was developed, exploring the safety and efficacy of the addition of bevacizumab to standard chemoradiation (Table 3) [63]. Between 2006 and 2009, a total of 60 patients were enrolled. The median follow-up was 12.4 months (range: 4.6–31.4 months). Most patients had FIGO stage 2B (63%) disease and with a Zubrod performance status (PS) of 0 (67%). Eighty percent of cases were squamous. There were no treatment-related serious AEs. More recently, oncologic outcomes were published, with 81% 3-year OS and a 23% locoregional failure rate [64].
Another Phase II clinical trial exploring the combination of cisplatin 50 mg/m2 day 1 + topotecan 0.75 mg/m2 days 1, 2, 3 + bevacizumab 15 mg/kg day 1 on a 21-day cycle was recently published [65]. A total of 27 patients with persistent or recurrent cervical cancer, with no prior chemotherapy for recurrence, were enrolled. The 6-month PFS was 59% (80% CI: 46–70%), with median PFS and OS of 7.1 months and 13.2 months, respectively. Unfortunately, grade 3–4 hematologic toxicity was common (thrombocytopenia 82%, leukopenia 74%, anemia 63%, neutropenia 56%) on this treatment regimen. The majority of patients (78%) required unanticipated hospital admissions for supportive care and/or management of toxicities.
GOG protocol 240
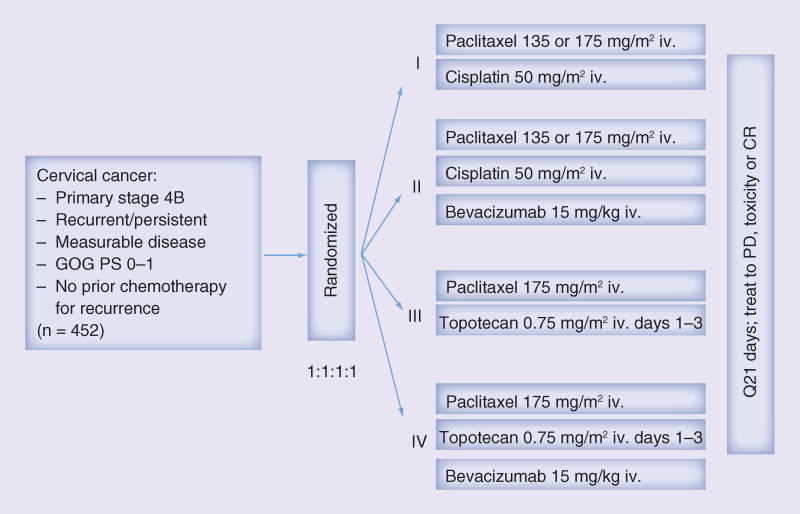
With publication of these Phase II studies, advancement of bevacizumab into the Phase III arena was a scientific priority. GOG protocol 240, a four-arm, prospective, randomized trial exploring platinum and nonplatinum doublets with and without the antiangiogenic agent bevacizumab, was designed and met its accrual goal in less than 3 years (Figure 4: GOG 240 schema) [21].
Figure 4.
Gynecologic Oncology Group protocol 240 schema.
CR: Complete response; GOG: Gynecologic Oncology Group; iv.: Intravenous; PD: Progressive disease; PS: Performance status; Q21 days: Every 21 days.
From April 2009 to January 2012, the trial accrued 452 patients. Over 220 patients were treated with each of the chemotherapy backbones, and patients were well matched for histology (p = 0.308), ethnicity (p = 0.800) and disease status: recurrent versus persistent versus advanced (p = 0.298). The majority of patients had a GOG PS of 0 (PS 0–1 required for enrollment). A total of 75% of the entire study group had previously received platinum and this was evenly distributed between the two backbones (p = 0.666). The topotecan + paclitaxel arm was shown to not be superior or inferior to the cisplatin + paclitaxel arm (HR: 1.20; 95% CI: 0.82–1.76). Median OS in the topotecan containing doublet was 12.5 months versus 15 months in the cisplatin + paclitaxel arm. These results were interpreted as indicating that the nonplatinum chemotherapy doublet was not superior to cisplatin + paclitaxel for efficacy.
The investigators showed a significant improvement in OS in the bevacizumab-containing arms relative to chemotherapy alone (17 months vs 13.3 months, respectively; HR: 0.71; 95% CI: 0.54–0.95; p = 0.0035). significant improvement in PFS was also identified (8.2 months bevacizumab-containing arms and 5.9 months in the chemotherapy alone arms; HR: 0.67; 95% CI: 0.54–0.82; p = 0.0002). Exploratory subanalysis further indicated the beneficial effects of bevacizumab in patients with prior platinum exposure, recurrent or persistent disease and squamous histology. Importantly, the benefits of bevacizumab persisted in patients with recurrent disease in a previously irradiated field, which was hypothesized to be relatively hypoxic. These findings represent the first time a targeted antiangiogenic agent has shown an improvement in OS in patients with gynecologic cancer.
More recently, the final protocol-specified OS analysis for GOG 240 was presented at the Annual Meeting of the European Society of Medical Oncology in Madrid, Spain (2014) following acceptance as a late breaking abstract [66]. As of 7 March 2014, 348 deaths had occurred and the regimens administering bevacizumab continued to demonstrate a significant improvement in OS over chemotherapy alone: 16.8 months versus 13.3 months; HR: 0.765 (95% CI: 0.62–0.95; p = 0.0068). Overall, a total of 20 patients who had been treated on the chemotherapy alone arms went on to receive salvage therapy with bevacizumab.
Bevacizumab & QoL on GOG 240
As with all new drugs, the therapeutic benefits are weighed against possible toxicity and an impact on QoL. In GOG 240, eight subjects (four in each arm) experienced a treatment-related death. Within the bevacizumab-containing arms, there was an increase in grade ≥3 GI and GU fistula (n = 5), as well as grade ≥2 hypertension, grade ≥4 neutropenia and grade ≥3 thrombocytopenia.
The full QoL data were presented at the European Society of Medical Oncology Annual Meeting (Amsterdam, The Netherlands, 2013) [67]. The primary and coprimary QoL end points were measured by the Trial Outcome Index of the Functional Assessment of Cancer Therapy-Cervix (FACT-Cx TOI) and FACT/GOG-Neurotoxicity subscale, respectively. The secondary QoL end point was worst pain in 24 h by the Brief Pain Inventory. The QoL parameters were assessed precycle 1, 2, and 5 and at 6 and 9 months postcycle 1. Of the 452 patients enrolled on study, 96% completed baseline QoL assessments, and 63% completed assessment at 9 months postcycle 1. The completion rates were not statistically different among the treatment regimens at any of the five assessment points (p = 0.67).
The fitted mixed model estimates indicated that patients receiving chemotherapy + bevacizumab reported 1.2 points (98.75% CI: −4.1–1.7; p = 0.3) lower on average in the FACT-Cx TOI scores than those treated with chemotherapy alone. The fitted MEMD (mixed effects mixed distribution) model estimates indicated patients treated with chemotherapy + bevacizumab were less likely to report neurotoxic symptoms (OR: 0.58; 98.75% CI: 0.29–1.17; p = 0.053). Severity of reported neurotoxic symptoms did not differ between the two groups (p = 0.7). The fitted MEMD model estimates also indicated that both groups had similar odds of complaining of pain (OR: 0.89; 95% CI: 0.36–1.42; p = 0.7) and reported similar severity of pain when they had it (p = 0.16).
The above results indicate that the improvement in OS and PFS attributed to the addition of bevacizumab to the doublet chemotherapy backbone was not accompanied by a significant deterioration in QoL.
Predictors of response: application of the Moore criteria to the GOG 240 population
In 2010, Moore et al. developed a model of prognostic factors predictive of (non-) response to chemotherapy in patients with advanced stage or recurrent cervical cancer [68]. GOG protocols 110, 169 and 179 were used for model development (training data set) and patients from GOG 149 were used for model validation (testing data set).
A total of 428 patients with advanced cervical cancer who received a cisplatin-containing combination in GOG protocols 110, 169 and 179 were evaluated for baseline clinical characteristics and multivariate analysis was conducted to identify factors independently prognostic/predictive of response using a logistic regression model. As detailed above, predictive model was developed and externally validated using an independent protocol (GOG 149). Multivariate analysis identified five factors (African–American, PS >0, pelvic disease, prior radiosensitizer and time interval from diagnosis to first recurrence <1 year) independently prognostic of poor response [68]. When patients were classified into three risk groups (low risk: 0–1 factor; mid risk: 2–3 factors; high risk: 4–5 factors), patients with four to five risk factors were estimated to have a response rate of only 13%, and median PFS and OS of 2.8 months and 5.5 months, respectively. The accuracy of the index was supported by both internal and external data sets.
Given the above findings, these prognosticators where evaluated prospectively as an exploratory end point in GOG 240 and the results were presented at the 2014 Society of Gynecologic Oncology Annual Meeting (FL, USA) [69]. Importantly, for the entire GOG 240 study population, the Moore criteria were prospectively validated. Interestingly, those patients with higher risk stratification (i.e., mid-risk and high-risk) appeared to derive the greatest benefit through the incorporation of antiangiogenesis therapy.
Proposed mechanisms of AEs
Hypertension, thromboembolic events, and fistula were observed more frequently among women receiving chemotherapy plus bevacizumab in the GOG 240 population. While the mechanism of anti-VEGF therapy-induced hypertension has not been fully elucidated, nitric oxide pathway inhibition, rarefaction and oxidative stress may be critical in its pathogenesis [70]. Although nephrotic syndrome was not observed in GOG 240, glomerular injury may develop with diminished effect of VEGF in maintaining the filtration barrier.
Because VEGF has a maintenance role for normal endothelium function, thromboembolic events may result from endothelial cell pertubations induced by anti-VEGF therapy, resulting in nonphysiologic endothelial cell apoptosis [71]. Abnormal apoptosis of endothelial cells can lead to exposure of the highly prothrombotic basement membrane. Alternatively, because VEGF signaling is essential for the production of platelet inhibitors prostaglandin I-2 and nitric oxide, the prothrombotic effect of bevacizumab may also derive from a platelet-dependent mechanism [71]. Sequestration of VEGF depletes prostaglandin I-2 and nitric oxide, resulting in platelet activation.
Inhibition of VEGF signaling has been shown to reduce vascular density in a variety of tissues in animal models, including small intestinal villi, pancreatic islets, thyroid and adrenal cortex [72]. The trigger of vascular regression may manifest by local detachment or death of endothelial cells, with excessive VEGF inhibition on the capillary beds of small intestinal villi contributing directly to gastrointestinal perforation by inducing regression of normal blood vessels. Risk factors for gastrointestinal perforation among patients receiving bevacizumab for colorectal cancer include primary tumor intact, recent history of sigmoidoscopy or colonoscopy and/ or previous adjuvant radiotherapy [73]. Among women with newly diagnosed ovarian cancer, risk factors for gastrointestinal AEs include a history of treatment for inflammatory bowel disease and bowel resection at primary surgery [74]. It is possible that the pathophysiology concerning intestinal vascular regression and some shared risk factors (e.g., prior pelvic radiotherapy) may contribute to the development of fistula among women with advanced cervical cancer treated with anti-VEGF therapy.
Conclusion
As our understanding of tumor biology and the tumor microenvironment progresses, therapeutics have analogously evolved from traditional cytotoxic molecules to novel monoclonal antibodies, peptibody conjugates, targeted biologic therapies and most recently immunotherapeutics. The importance of GOG 240 centers on the fact that it represents the first time a targeted agent has reached its primary end point, improving OS, in patients with gynecologic cancer. As detailed above, this survival benefit was not associated with a decrement in QoL, and additional studies are ongoing to help identify potential predictors of response, including the exploration of gene signatures [71]. Unlike patients with advanced stage ovarian or mBC, where salvage therapy commonly translates into partial or complete response, patients with metastatic or recurrent cervical have failed to show meaningful response to multimodal therapy in prior studies. With the publication of GOG 240, it is anticipated that patients with what was traditionally viewed as poor prognosis cervical cancer may achieve durable remission, improving QoL, and potentially opening the door to alternate novel therapies.
EXECUTIVE SUMMARY.
Angiogenesis is essential for tumor growth & has been identified as a therapeutic target in solid malignancies
Biologically, angiogenesis (abnormal vascularity) imparts an aggressive course in colposcopic exam of the cervix.
US FDA approval of bevacizumab in colorectal, renal, non-small-cell lung cancer and glioblastoma.
FDA approval anticipated in metastatic cervical cancer following publication of Gynecologic Oncology Group (GOG) 240.
Bevacizumab, a monoclonal antibody directed against VEGF (ligand) was developed & tested in various solid tumors
It is a recombinant humanized monoclonal IgG1 antibody (93% human, 7% murine sequences – molecular weight 149 kDa).
Pharmacokinetics are described by a two-compartment model.
Binding of the IgG to the FcRn receptor results in protection from cellular metabolism and the long terminal half-life.
Metabolism of bevacizumab is more rapid in patients with low albumin and higher volume of disease.
Phase II clinical trials were conducted, & demonstrated the safety & efcacy of bevacizumab in patients with cervical cancer
Response rate of 33–35% in a heavily pretreated population.
Only one grade 4 adverse event in the combined studies.
GOG 240
Accrued 452 patients with advanced stage, metastatic or recurrent cervical cancer over a 3-year period.
Showed both progression-free survival and overall survival advantage with the addition of bevacizumab to cytotoxic chemotherapy.
GOG 240 represents the first time a targeted agent has reached its primary end point, improving overall survival, in patients with gynecologic cancer with practice changing implications.
No decrement in quality of life.
Moore prognostic criteria applied to GOG 240, indicating that patients with worst prognostic classifcation may benefit most from treatment with bevacizumab.
Conclusion
Patients with advanced stage cervical cancer had limited therapeutic options prior to publication of GOG 240.
Bevacizumab is anticipated to receive FDA approval for the treatment of recurrent, metastatic or advanced stage cervical cancer in the USA (already received in EMA).
Developing biomarkers predictive of response is critical, and application of a proangiogenic gene signature to the GOG 240 population is implicit.
Acknowledgments
KS Tewari has served on Advisory Boards for Genentech (travel and accommodation). RN Eskander was recently appointed to Genentech’s Speaker’s Bureau for Ovarian and Cervical Cancer. This study was supported by the Ruth L Kirschstein NRSA Institutional Training Research Grant, 2T32 CA06039611.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Greer BE, Koh WJ, Abu-Rustum NR, et al. Cervical cancer. J. Natl Compr. Canc. Netw. 2010;8:1388–1416. doi: 10.6004/jnccn.2010.0104. [DOI] [PubMed] [Google Scholar]
- 4.Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing J. Cis-platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a Phase II study of the Gynecologic Oncology Group. Cancer. 1981;48:899–903. doi: 10.1002/1097-0142(19810815)48:4<899::aid-cncr2820480406>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.McGuire WP3rd, Arseneau J, Blessing JA, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J. Clin. Oncol. 1989;7:1462–1468. doi: 10.1200/JCO.1989.7.10.1462. [DOI] [PubMed] [Google Scholar]
- 6.Fracasso PM, Blessing JA, Wolf J, Rocereto TF, Berek JS, Waggoner S. Phase II evaluation of oxaliplatin in previously treated squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol. Oncol. 2003;90:177–180. doi: 10.1016/s0090-8258(03)00253-1. [DOI] [PubMed] [Google Scholar]
- 7.Thigpen T, Blessing JA, Gallup DG, Maiman M, Soper JT. Phase II trial of mitomycin-C in squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecol. Oncol. 1995;57:376–379. doi: 10.1006/gyno.1995.1157. [DOI] [PubMed] [Google Scholar]
- 8.Sutton GP, Blessing JA, Adcock L, Webster KD, DeEulis T. Phase II study of ifosfamide and mesna in patients with previously-treated carcinoma of the cervix: a Gynecologic Oncology Group study. Invest. New Drugs. 1989;7:341–343. doi: 10.1007/BF00173765. [DOI] [PubMed] [Google Scholar]
- 9.Look KY, Blessing JA, Levenback C, Kohler M, Chafe W, Roman LD. A Phase II trial of CPT-11 in recurrent squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol. Oncol. 1998;70:334–338. doi: 10.1006/gyno.1998.5129. [DOI] [PubMed] [Google Scholar]
- 10.Schilder RJ, Blessing JA, Morgan M, Mangan CE, Rader JS. Evaluation of gemcitabine in patients with squamous cell carcinoma of the cervix: a Phase II study of the Gynecologic Oncology Group. Gynecol. Oncol. 2000;76:204–207. doi: 10.1006/gyno.1999.5671. [DOI] [PubMed] [Google Scholar]
- 11.Bookman MA, Blessing JA, Hanjani P, Herzog TJ, Andersen WA. Topotecan in squamous cell carcinoma of the cervix: a Phase II study of the Gynecologic Oncology Group. Gynecol. Oncol. 2000;77:446–449. doi: 10.1006/gyno.2000.5807. [DOI] [PubMed] [Google Scholar]
- 12.Curtin JP, Blessing JA, Webster KD, et al. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2001;19:1275–1278. doi: 10.1200/JCO.2001.19.5.1275. [DOI] [PubMed] [Google Scholar]
- 13.McGuire WP, Blessing JA, Moore D, Lentz SS, Photopulos G. Paclitaxel has moderate activity in squamous cervix cancer. A Gynecologic Oncology Group study. J. Clin. Oncol. 1996;14:792–795. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 14.Garcia AA, Blessing JA, Vaccarello L, Roman LD. Phase II clinical trial of docetaxel in refractory squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Am. J. Clin. Oncol. 2007;30:428–431. doi: 10.1097/COC.0b013e31803377c8. [DOI] [PubMed] [Google Scholar]
- 15.Muggia FM, Blessing JA, Waggoner S, et al. Evaluation of vinorelbine in persistent or recurrent nonsquamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol. Oncol. 2005;96:108–111. doi: 10.1016/j.ygyno.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J. Clin. Oncol. 2002;20:1832–1837. doi: 10.1200/JCO.2002.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Long HJ3rd, Bundy BN, Grendys EC, Jr, et al. Randomized Phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J. Clin. Oncol. 2005;23:4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 18•.Monk BJ, Sill MW, McMeekin DS, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J. Clin. Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. Established cisplatin and paclitaxel as the standard chemotherapy backbone for future therapeutic trials in patients with advanced stage, recurrent or persistent cervical cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J. Clin. Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 20.Omura GA, Blessing JA, Vaccarello L, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J. Clin. Oncol. 1997;15:165–171. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 21••.Tewari KS, Sill MW, Long HJ, 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. First clinical trial to results in the approval of a targeted agent, bevacizumab, in the treatment of a gynecologic cancer. Resulted in US FDA approval of bevacizumab in this disease setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eskander RN, Tewari KS. Emerging treatment options for management of malignant ascites in patients with ovarian cancer. Int. J. Womens Health. 2012;4:395–404. doi: 10.2147/IJWH.S29467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roche Bevacizumab Data Sheet (DS 140207) 2014 www.medsafe.govt.nz.
- 24.Ferrara N, Hillan K, Gerber H, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 25.Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 27.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 28.Sandler A, Gray R, Perry M, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 29.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 30.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J. Clin. Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 31.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind Phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 33.Oza AM, Perren TJ, Swart AM, et al. ICON7: Final overall survival results in the GCIG Phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer. Presented at: European Society of Medical Oncology Annual Meeting; Amsterdam, The Netherlands. 30 September 2013. [Google Scholar]
- 34.Perren TJ, Swart AM, Pfisterer J, et al. A Phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 35.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 36.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled Phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eskander RN, Tewari KS. Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma—mechanistics, review of Phase III randomized clinical trials, and regulatory implications. Gynecol. Oncol. 2014;132:496–505. doi: 10.1016/j.ygyno.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Eskander RN, Randall LM. Bevacizumab in the treatment of ovarian cancer. Biologics. 2011;5:1–5. doi: 10.2147/BTT.S13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized Phase III trial. J. Clin. Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 40•.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. Discusses the limitations of overall survival as an end point in the modern era of clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Ocejo O, Viloria-Petit A, Bequet-Romero M, Mukhopadhyay D, Rak J, Kerbel RS. Oncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene. 2000;19:4611–4620. doi: 10.1038/sj.onc.1203817. [DOI] [PubMed] [Google Scholar]
- 42.Toussaint-Smith E, Donner DB, Roman A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is suffcient to alter the expression of angiogenic factors. Oncogene. 2004;23:2988–2995. doi: 10.1038/sj.onc.1207442. [DOI] [PubMed] [Google Scholar]
- 43.Coussens LM, Hanahan D, Arbeit JM. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am. J. Pathol. 1996;149:1899–1917. [PMC free article] [PubMed] [Google Scholar]
- 44.Eskander RN, Tewari KS. Targeting angiogenesis in advanced cervical cancer. Ther. Adv. Med. Oncol. 2014;6:280–292. doi: 10.1177/1758834014543794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl. 3):11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 46.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 47.Cooper RA, Wilks DP, Logue JP, et al. High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin. Cancer Res. 1998;4:2795–2800. [PubMed] [Google Scholar]
- 48.Kudelka AP, Levy T, Verschraegen CF, et al. A Phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin. Cancer Res. 1997;3:1501–1505. [PubMed] [Google Scholar]
- 49.Kudelka AP, Verschraegen CF, Loyer E. Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N. Engl. J. Med. 1998;338:991–992. doi: 10.1056/NEJM199804023381412. [DOI] [PubMed] [Google Scholar]
- 50.Cheng WF, Chen CA, Lee CN, Wei LH, Hsieh FJ, Hsieh CY. Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet. Gynecol. 2000;96:721–726. doi: 10.1016/s0029-7844(00)01025-5. [DOI] [PubMed] [Google Scholar]
- 51.Guidi AJ, Abu-Jawdeh G, Berse B, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J. Natl Cancer Inst. 1995;87:1237–1245. doi: 10.1093/jnci/87.16.1237. [DOI] [PubMed] [Google Scholar]
- 52.Lee CM, Shrieve DC, Zempolich KA, et al. Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol. Oncol. 2005;99:415–421. doi: 10.1016/j.ygyno.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 53.Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br. J. Cancer. 2000;83:620–625. doi: 10.1054/bjoc.2000.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva-Filho AL, Traiman P, Triginelli SA, et al. Association between CD31 expression and histopathologic features in stage IB squamous cell carcinoma of the cervix. Int. J. Gynecol. Cancer. 2006;16:757–762. doi: 10.1111/j.1525-1438.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 55.Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24:23–39. doi: 10.2165/11530560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 57.Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin. Pharmacokinet. 2013;52:83–124. doi: 10.1007/s40262-012-0027-4. [DOI] [PubMed] [Google Scholar]
- 59.Wright JD, Viviano D, Powell MA, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol. Oncol. 2006;103:489–493. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Hale G, Rebello P, Brettman LR, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104:948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 61•.Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J. Clin. Oncol. 2009;27:1069–1074. doi: 10.1200/JCO.2008.18.9043. Positive Phase II study that catalyzed the synthesis and completion of GOG 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tewari KS, Monk BJ. Gynecologic oncology group trials of chemotherapy for metastatic and recurrent cervical cancer. Curr. Oncol. Rep. 2005;7:419–434. doi: 10.1007/s11912-005-0007-z. [DOI] [PubMed] [Google Scholar]
- 63.Schefter TE, Winter K, Kwon JS, et al. A Phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:1179–1184. doi: 10.1016/j.ijrobp.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 64.Schefter T, Winter K, Kwon JS, et al. RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:101–105. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 65.Zighelboim I, Wright JD, Gao F, et al. Multicenter Phase II trial of topotecan, cisplatin and bevacizumab for recurrent or persistent cervical cancer. Gynecol. Oncol. 2013;130:64–68. doi: 10.1016/j.ygyno.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.KS Tewari MS, Penson R, Huang H, et al. Final overall survival analysis of the Phase III randomized trial of chemotherapy with and without bevacizumab for advanced cervical cancer: a NRG Oncology – Gynecologic Oncology Group Study. Presented at: 2014 Annual Meeting of the European Society of Medical Oncology; Madrid, Spain. 28 September 2014; (Late Breaking Abstract #26). Final overall survival analysis of GOG 240 showing a sustained benefit in the bevacizumab containing arms. [Google Scholar]
- 67••.Penson RT, Huang HQ, Wenzel LB, et al. Bevaiczumab for advanced cervical cancer: patient-reported outcomes of a randomized, Phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240) Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)70004-5. (Epub ahead of print). Results describe that the overall survival benefit associated with bevacizumab did not result in a significant decline in quality of life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD. Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study. Gynecol. Oncol. 2010;116:44–49. doi: 10.1016/j.ygyno.2009.09.006. Established high-risk criteria predictive of poor response to therapy in patients with advanced cervical cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Tewari KS, Sill MW, Monk BJ, et al. Prospective validation of pooled clinical prognostic factors in patients with recurrent and advanced cervical cancer: a Gynecologic Oncology Group (GOG) study. Gynecol. Oncol. 2014;133:59–60. (SGO Abstract 143). Discusses the application of the Moore criteria (reference 67 above) to GOG 240. [Google Scholar]
- 70.Tewari KS, Sill MW, Moore DH, et al. High-risk patients with recurrent/advanced cervical cancer may derive the most benefit from anti-angiogenesis therapy: a Gynecologic Oncology Group (GOG) study. Gynecol. Oncol. 2014;133:207. (Abstract 144) [Google Scholar]
- 71••.Gourley C, Perren TJ, Paul J, et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J. Clin. Oncol. 2014;32(Suppl. 5) Abstract 5502 Study aimed at identifying gene signatures predictive of response to antiangiogenic therapy in patients with ovarian cancer. [Google Scholar]
- 72.Kamba T, Tam BY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 73.Saif MW, Elfiky A, Salem RR, et al. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann. Surg. Oncol. 2007;14:1860–1869. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 74.Burger RA, Brady MF, Bookman MA, et al. Risk factors for GI adverse events in a Phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2014;32:1210–1217. doi: 10.1200/JCO.2013.53.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]