Figure 3. Neutralization of a panel of viruses and glycovariant viruses suggests that CAP256.25 Ab CDRH2 residues interact with sialic acid likely on N156 glycan.
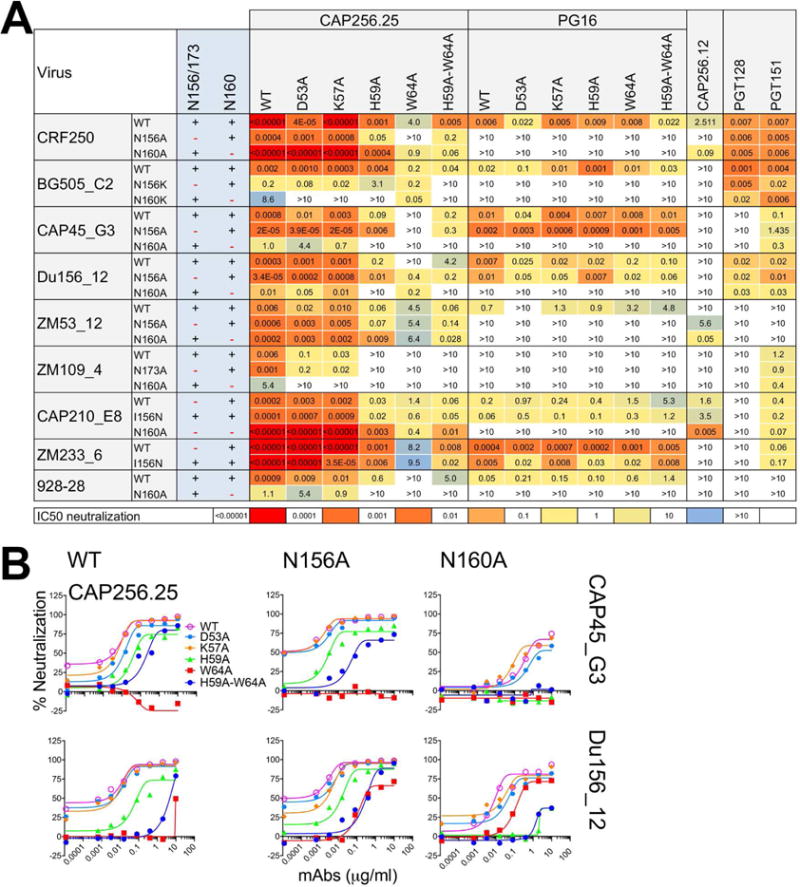
A. Neutralization of a panel of viruses including N156 or 173A and N160A variants by CAP256.25 and PG16 Abs and their CDRH2 variants. Neutralization heat maps based on the IC50 titers show the effect of glycan removals on antibody neutralizing activity. PGT128, an N332-V3 Ab and PGT151, a gp120-41 interface Ab, were used as controls. B. Neutralization titration of CAP256.25 WT Ab and its CDRH2 substituted variants against two representative viruses, CAP45_G3 and Du156.12 and their glycan eliminated N156A, N160A variants.
