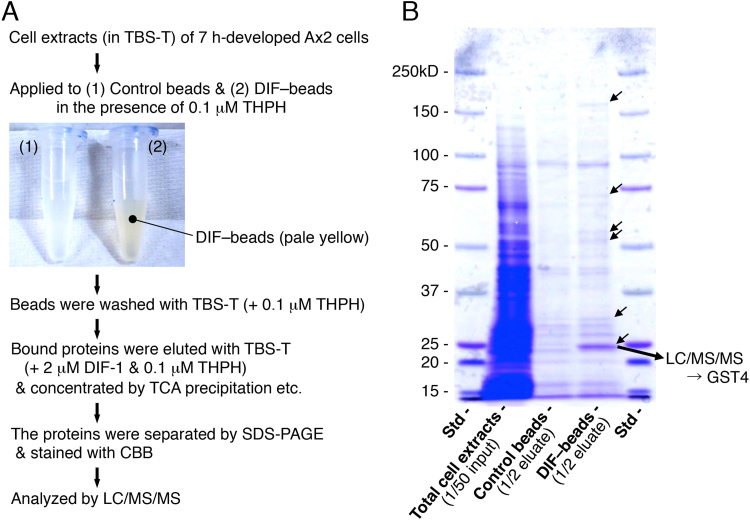
Fig. 2.
(A) Scheme for the identification of DIF-binding proteins in D. discoideum. Ax2 cells were developed on agar plates for 7 h, lysed in 0.05% Tween 20 in Tris-buffered saline (TBS-T), and incubated with control beads or DIF–beads in the presence of 0.1 μM THPH to reduce non-specific protein binding to beads. After beads were washed with TBS-T containing THPH, the bound proteins were eluted by using TBS-T containing 2 μM DIF-1. The affinity-purified proteins were separated by SDS-PAGE and stained with Coomassie brilliant blue. The proteins that were specifically present in the DIF-beads eluate were identified by using LC/MS/MS. (B) Photo of the SDS-PAGE gel stained with Coomassie brilliant blue. Total cell extracts, eluates of control and DIF–beads, and molecular mass standards (Std) were subjected to SDS-PAGE, and the resulting gel was stained; arrows indicate a major band and minor bands unique to the DIF-beads lane. The ~25-kD protein band present in the DIF-beads lane was harvested, and the proteins in the band were analyzed by LC/MS/MS. Ultimately, the major protein in the band was identified as the glutathione S-transferase GST4.