Abstract
A young man presented with the severe right upper limb swelling following a heavy weight lifting that was thought to be caused by a biceps tendon rupture. However, subsequent investigations confirmed the diagnosis of Paget-Schroetter syndrome that was associated with an incidental pulmonary embolism. The patient underwent a successful thrombolysis followed by a surgical thoracic outlet decompression. Overall, the patient has made a good recovery.
Keywords: Vascular Surgery, Pulmonary embolism, Interventional radiology
Background
Paget-Schroetter syndrome (PSS) is an infrequent and disabling condition with reported incidence in 2 per 100 000 people per year.1 2Pulmonary embolism (PE) is potentially the most serious complication following PSS and has been reported up to 10% of presentations.3 Furthermore, PE is associated with the future risk of developing chronic thromboembolic pulmonary hypertension (CTEPH) that is associated with a significant morbidity and mortality.3 4 Hence, suboptimal treatment of PSS and its complications may lead to devastating consequences. In the presented case, the diagnosis of PE was established incidentally. Therefore, we report this case to highlight the importance of a prompt vascular referral and early imaging.
Case presentation
An 18-year-old man presented with 10 days history of the severe right upper limb swelling, bruising and discomfort following an exercise involving heavy weight lifting. There was no medical history and the patient denied any previous limb injury or surgical intervention. The patient was a non-smoker and had no allergies.
Examination of upper limbs revealed the full complement of all pulses, normal sensation and power. There was a significant swelling and dusky discolouration of the entire right upper limb. Examination of the respiratory system revealed reduced breath sounds at the right lung base. The cardiovascular assessment was normal. A provisional diagnosis of the biceps tendon rupture was made. The patient was discharged from an emergency department with a follow-up ultrasound scan that was performed 72 hours later.
Investigations
Ultrasound scan excluded musculoskeletal trauma and revealed the right subclavian vein (SCV) occlusive thrombus extending medially to the confluence with the internal jugular vein. The above finding prompted an urgent CT that revealed no obvious cause of the SCV thrombosis but demonstrated the right lower lobe segmental PE with a small localised infarct. The patient underwent an emergency venography followed by a catheter-directed thrombolysis.
Treatment
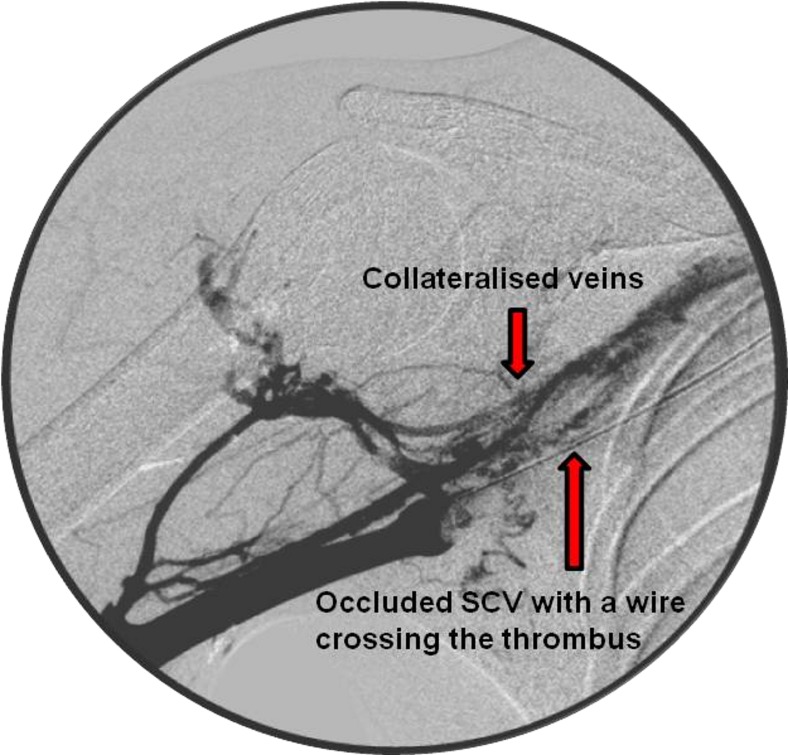
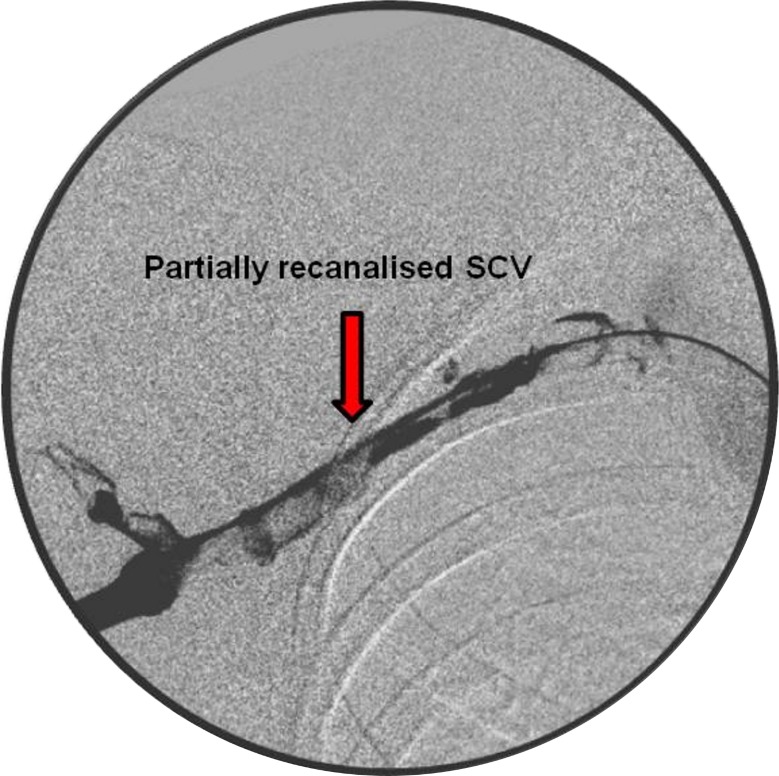
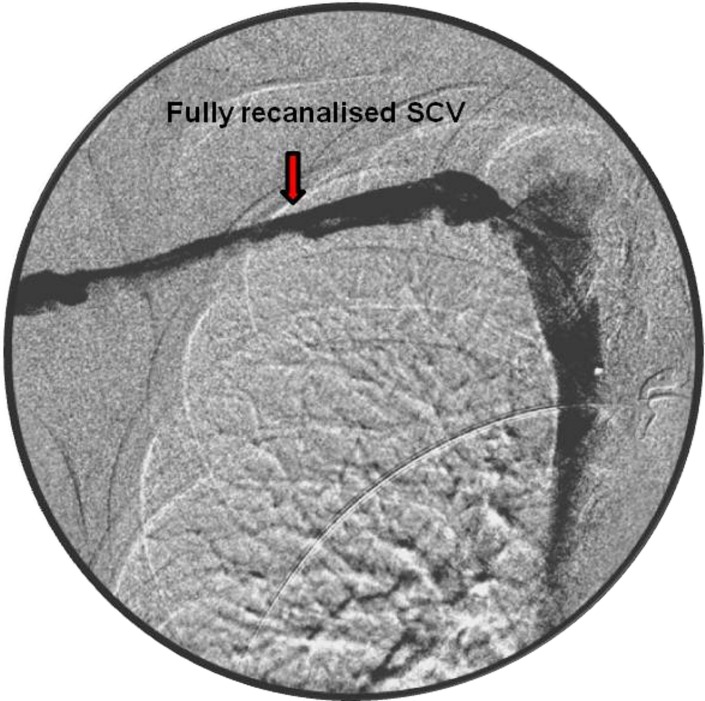
A 4 French sheath was placed in the basilic vein, and venogram confirmed the presence of a long thrombus within the SCV (figure 1). The occlusive thrombus was crossed with 20 cm long thrombolytic catheter and boluses of alteplase (5 mg) with heparin (500 U) were administered. Subsequently, infusion with alteplase 1 mg/hour and heparin 1 mL/hour were continued for 24 hours. A check venogram showed a persistently high load of thrombus in the SCV (figure 2). Hence, the catheter's position was readjusted and the intravenous anticoagulants were continued with regular clotting profile checks. At 48 hours, repeated venogram revealed a full recanalisation of the venous system (figure 3). The thrombolysis was discontinued, and the patient was started on low-molecular-weight heparin which was continued until a surgical decompression of his thoracic outlet was performed. An excision of the right first rib was carried out at 2 weeks via the transaxillary root. Postoperatively, the patient was started on rivaroxaban (15 mg two times per day) for 3 months to treat an underlying PE.
Figure 1.
An occluded subclavian vein (SCV) on diagnostic venogram.
Figure 2.
Partial subclavian vein (SCV) recanalisation after 24 hours of thrombolysis.
Figure 3.
Fully recanalised subclavian vein (SCV) post 48 hours of thrombolysis.
Outcome and follow-up
There were no complications related to the endovascular treatment and open surgery. After a period of routine follow-up, the patient was discharged.
Discussion
PSS defines an acute thrombosis of the axillosubclavian vein (AXV-SCV) caused by compression in the narrow costoclavicular space at the thoracic outlet that leads to stasis of the flow.5 6 A strenuous and repetitive upper extremity movements lead to a cyclical endothelial microtrauma that induces thrombosis, intimal hyperplasia, inflammation and fibrosis.5 Complications include recurrent thrombosis, post-thrombotic syndrome and PE.3 The latter has been reported in up to 10% of patients, although the actual numbers may be higher owing to an asymptomatic presentation of PE in young adults.3 7 Furthermore, an acute episode of PE (with no previous episodes) is linked with a 4% risk of developing CTEPH within 2 years of an index event that is associated with a significant morbidity and mortality.4 Moreover, younger age and recurrent PE significantly increase the risk of developing this condition.4 More importantly, even lethal cases have been reported.7 Therefore, the suboptimal treatment of PSS may lead to devastating consequences in this young group of otherwise healthy individuals. Post-thrombotic syndrome is the most common complication after PSS. It affects up to 50% of patients and manifests as a myriad of symptoms such as chronic and disabling limb swelling, heaviness and pain.3 5 This, in turn, leads to a reduced quality of life and work-related problems.2 Diagnosis is usually established by a combination of clinical and radiological findings. Radiological confirmation can be achieved by duplex ultrasound scan, CT venography (CTV), magnetic resonance venography (MRV) and formal diagnostic venography. A duplex ultrasound scan is a simple, non-invasive and easily available first-line imaging modality that allows identifying AXV-SCV thrombosis.8 Whereas, CTV and MRV provide an excellent anatomical resolution with high sensitivity and specificity.3 5 The choice depends on the availability of these imaging modalities and urgency of the clinical presentation. The role of venography is crucial in confirming the diagnosis, but most importantly it allows delivering catheter-directed thrombolysis that is an essential part of the treatment algorithm. Although no level I evidence exists regarding an optimal treatment approach, multiple studies have demonstrated superior results following thrombolysis with thoracic outlet decompression (TOD) when compared with a conservative approach (anticoagulation alone) that leads to recurrent thromboses and chronic limb disability.1 2 4 5 9 10
Locally delivered lysis is highly effective up to 4 weeks from the presentation and should be followed by TOD by the means of first rib excision via the transaxillary, supra or infraclavicular routes.1 3 TOD is safe with few complications when performed by an experienced surgeon and provides good to excellent postoperative functional outcomes.1 9
The role of the preoperative anticoagulation is to limit propagation of the thrombus and to prevent PE.11 However, the requirement for anticoagulants in a postoperative period remains debatable in cases without coincidental PE or diagnosis of the thrombophilic disorder. Some advocate continuing anticoagulation for 3 or 6 months following TOD.11 Arguably, this may help to maintain patency of the venous collaterals. However, no effect has been found on rethrombosis rates or functional scores with the regular use of anticoagulants.1
This case highlighted that PSS is a relatively unique disorder and if treated inadequately can lead to a significant morbidity and limb disability. Hence, there is a need to increase awareness among medical staff about the effort induced AXV-SCV thrombosis, its management and the potential for serious complications, such as PE and CTEPH.
Learning points.
Paget-Schroetter syndrome is a rare disorder that can be complicated by pulmonary embolism.
Treatment includes thrombolysis and thoracic outlet decompression.
Prompt vascular referral is vital to ensure early diagnosis and treatment.
Footnotes
Contributors: JK performed literature search and drafted the manuscript. JS checked and approved the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Taylor JM, Telford RJ, Kinsella DC, et al. . Long-term clinical and functional outcome following treatment for Paget-Schroetter syndrome. Br J Surg 2013;100:1459–64. 10.1002/bjs.9279 [DOI] [PubMed] [Google Scholar]
- 2.Khan SN, Stansby G. Current management of Paget-Schroetter syndrome in the UK. Ann R Coll Surg Engl 2004;86:29–34. 10.1308/003588404772614650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard JD, Gains PA. Vascular disorders of the upper limb Vascular and endovascular surgery. 4th ed Edinburgh: Elsevier, 2009. [Google Scholar]
- 4.Pengo V, Lensing AW, Prins MH, et al. . Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257–64. 10.1056/NEJMoa032274 [DOI] [PubMed] [Google Scholar]
- 5.Alla VM, Natarajan N, Kaushik M, et al. . Paget-schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med 2010;11:358–62. [PMC free article] [PubMed] [Google Scholar]
- 6.Parvin SD. Paget Schroetter syndorme Rare vascular disorders: a practical guide for the vascular specialist.: tfm publishing limited, 2005. [Google Scholar]
- 7.Black MD, French GJ, Rasuli P, et al. . Upper extremity deep venous thrombosis. underdiagnosed and potentially lethal. Chest 1993;103:1887–90. [DOI] [PubMed] [Google Scholar]
- 8.Ochoa VM, Yeghiazarians Y. Subclavian artery stenosis: a review for the vascular medicine practitioner. Vasc Med 2011;16:29–34. 10.1177/1358863X10384174 [DOI] [PubMed] [Google Scholar]
- 9.Azakie A, McElhinney DB, Thompson RW, et al. . Surgical management of subclavian-vein effort thrombosis as a result of thoracic outlet compression. J Vasc Surg 1998;28:777–86. 10.1016/S0741-5214(98)70052-7 [DOI] [PubMed] [Google Scholar]
- 10.Ferrante MA. The thoracic outlet syndromes. Muscle Nerve 2012;45:780–95. 10.1002/mus.23235 [DOI] [PubMed] [Google Scholar]
- 11.Joffe HV, Goldhaber SZ. Upper-extremity deep vein thrombosis. Circulation 2002;106:1874–80. 10.1161/01.CIR.0000031705.57473.1C [DOI] [PubMed] [Google Scholar]