Abstract
Patients with acute lymphoblastic leukemia with minimal residual disease present at time of allogeneic hematopoietic stem cell transplant showed a trend for greater risk of relapse following transplant in this retrospective, single center study.
Allogeneic hematopoietic stem cell transplantation (HSCT) is highly effective for treating acute lymphoblastic leukemia (ALL). However, many ALL patients relapse after HSCT. There has been a continuing effort to improve identification of patients at high risk of relapse, with the goal of early intervention to improve outcome. In this retrospective analysis, we examined the impact of minimal residual disease (MRD) on the risk of hematologic relapse in 149 adult patients with ALL undergoing allogeneic HSCT in morphologic remission. MRD was assessed at time of HSCT and following HSCT. Patients with pre-transplant MRD had a trend for shorter progression-free survival (PFS) at 2 years compared with patients without MRD, nearing statistical significance, 28% vs. 47%, p=0.08, on univariate analysis. This trend remained on multivariate analysis with better PFS in patients without MRD at time of HSCT, hazard ratio (HR) 0.62 (95% CI 0.37, 1.04), p=0.07. Additionally, emergence of MRD post-HSCT was a strong predictor for overt hematologic relapse (HR 4, p<0.001) with a median latency interval of 3.8 months. These findings demonstrate the predictive value of monitoring for MRD peri-transplant in adult patients with ALL.
Keywords: Minimal residual disease, acute lymphoblastic leukemia, allogeneic hematopoietic stem cell transplantation, prognosis, relapse
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only effective therapy for patients with high-risk or relapsed acute lymphoblastic leukemia (ALL). Select patients with ALL who have received allogeneic HSCT have a significant survival advantage over patients without HSCT 1-6. However, substantial numbers of ALL patients still relapse after HSCT, mostly occurring within the first two years after transplant. There has been a continuing effort to improve the identification of patients at high risk for relapse after HSCT 7-11, with the goal of early therapeutic intervention to improve outcome.
Minimal residual disease (MRD) at the end of induction or induction/consolidation therapy is one of the most significant risk factors for subsequent disease relapse in ALL patients 12-20. Persistent MRD has become a significant indicator for HSCT or intensified chemotherapy 17, 21. The presence of MRD prior to HSCT is also a predictor for relapse, but has been less well-studied in adults compared with children 22, 23. In pediatric patients, the presence of MRD prior to HSCT is highly predictive for post-transplant relapse 24-28. The frequency of disease progression in children with MRD prior to HSCT is about 3-fold the frequency in children without MRD. However, the impact of MRD at time of HSCT in adults with ALL is less clear, with conflicting results. In reports by Bassan 21 and Spinelli and colleagues 29, MRD was assessed at time of transplant using polymerase chain reaction (PCR) amplification and patient leukemia specific probes, and was found to be a predictor for relapse. In contrast, in the UKALL XII/Eastern Cooperative Oncology Group (ECOG) 2993 study using similar methods to assess for MRD in 161 patients with B-lineage Philadelphia chromosome-negative ALL, the presence of MRD was not associated with a higher rate for relapse 21, 23. Interestingly, MRD at time of autologous HSCT was associated with a higher rate of relapse suggesting that the graft versus leukemia (GVL) effect was protective against MRD in the allogeneic HSCT setting.
Furthermore, there are only limited studies of the risk, or tempo, of progression in patients who demonstrate positive MRD soon after HSCT 30, 31. Using immunoglobulin and T-cell receptor rearrangements as clonal markers, Uzunel and colleagues showed that detectable MRD preceded relapse in 8 of 14 patients, with a median time of 5 months between first MRD detection and relapse 30. Regular MRD monitoring after transplant may offer an opportunity to detect emerging hematological relapse before overt hematological relapse, and thus provide a window for therapeutic intervention.
In this retrospective study, we investigated whether MRD before transplant as well as post-transplant had an association with patient outcomes, including overall (OS) and progression-free survival (PFS).
PATIENTS and METHODS
Patients
Uniform assessment of MRD by flow cytometric immunophenotyping (FCI) was established at our hospital in 2004. Therefore, our study cohort was limited to patients who received a first allogeneic HSCT at MD Anderson Cancer Center (MDACC) starting in February 2004 through October 2012. Patients needed to be in complete remission and have available MRD assessment by FCI within 30 days prior to HSCT. A total of 149 patients met these criteria and are included in the current study. The first assessment of MRD after SCT was done approximately 30 days after the procedure, and 135 patients had post-SCT MRD assessments. Patients were treated on clinical trials that were approved by the institutional review board (IRB), and written informed consent was obtained in accordance with the Declaration of Helsinki.
Donors
HLA typing for class I antigens was performed using standard serologic or low resolution molecular techniques, followed by confirmatory typing with high-resolution molecular typing using PCR for class I and II antigens for sibling donors; high-resolution molecular typing of class I and II antigens was performed for all unrelated donors. Peripheral blood stem cells were obtained from donors using standard mobilization protocols and apheresis techniques, with a target progenitor cell dose of 4 × 106 CD34+ cells/kg and minimal acceptable dose of 2 × 106 CD34+ cells/kg; bone marrow was used if peripheral blood could not be used. Stem cells from all related donors were collected at M. D. Anderson Cancer Center. Peripheral blood progenitor cells or bone marrow harvests from unrelated donors were obtained through the National Marrow Donor Program. All grafts were T lymphocyte replete.
Conditioning regimens
Patients received a variety of myeloablative transplant preparative regimens, based on available existing protocols at time of treatment. Conditioning intensity was defined according to CIBMTR criteria. 32 Myeloablative, radiation-based regimens were largely considered for patients younger than 50 years of age, and included cyclophosphamide (Cy) 60 mg/kg i.v. for 2 days, followed by 12 Gy of total body irradiation (TBI) 33, 34. Additionally, CyTBI was combined with rituximab 375 mg/m2 weekly for 4 doses 35, or alemtuzumab 10 mg for 5 doses, or TBI was combined with a single dose of etoposide at 60 mg/kg +/− rituximab. Non-TBI, myeloablative regimens included intravenous busulfan (Bu) at 130 mg/m2 infused daily for 4 days, either as a fixed dose per body surface area, or based on pharmacokinetic data derived from Bu test dose followed by melphalan (Mel) 70 mg/m2 for 2 doses 36 or followed by clofarabine 40 mg/m2 for 4 doses 37. Additionally, fludarabine (Flu) was administered at 25 mg/m2 daily ×5 doses followed by 2 daily doses of Mel 70 mg/m2.
Supportive care
Graft versus host disease (GVHD) prophylaxis consisted of a combination of tacrolimus and mini-dose methotrexate in all patients. Patients with matched unrelateddonors additionally received antithymocyte globulin for total dose of 4 mg/kg infused over three days. Central nervous system (CNS) prophylaxis after HSCT was recommended for patients with a prior history of CNS disease. Tyrosine kinase inhibitor (TKI) maintenance following HSCT was physician-based, and administered as feasible for patients with Ph+ ALL with adequate cell count recovery following HSCT. Institutional transplant guidelines for antimicrobial prophylaxis and blood transfusions were followed.
MRD assessment by FCI
MRD was assessed using multiparameter FCI with a sensitivity of 0.01%. Bone marrow aspirate specimens were analyzed using a panel of 10-21 markers, including in most cases CD10, CD13, CD15, CD19, CD20, CD22, CD33, CD34, CD38, CD45, CD58, and CD66c for B-lineage ALL38 and CD1, CD2, cytoplasmic CD3, surface CD3, CD4, CD5, CD7, CD8, CD10, CD13, CD33, CD34, CD45, CD56, HLA-DR and TdT for T-lineage ALL. Data were collected on 200,000 nucleated bone marrow cells. At the beginning of the study, samples were stained with 8-10 four-color tubes, and later samples were stained with 4 six-color tubes for B-lineage ALL and 5 seven-color tubes for T-lineage ALL. CD19 was included in each tube for gating in B-lineage ALL, along with CD34 to delineate the immature subset. Cytoplasmic CD3 staining was used for gating in T-lineage ALL, with surface CD3 also included in each tube to identify immature (surface CD3-negative) cells. MRD was scored as positive based on a distinct cluster of at least 20 cells on bivariate dot-plots, showing a significant difference in the level of expression (>=3-fold) of 2 or more antigens, in comparison to the known phenotype of benign immature B-cell precursors, or of mature marrow T and NK cells. Levels of MRD were reported as a fraction of total nucleated bone marrow cells. Samples for MRD were obtained within 30 days prior to HSCT, approximately 30 days following HSCT, and then every 3-6 months as feasible.
Clinical outcome variables and statistical methods
The disease stage at transplantation was defined using established criteria based on bone marrow morphology. Complete response (CR) was defined as normalization of cytogenetics and less than 5% bone marrow blasts. Response was documented as best response occurring after day 30 following HSCT. Molecular response measured by qPCR assay for BCR-ABL fusion transcripts developed at MDACC as previously described 39 was obtained when possible. Disease progression was defined as leukemic blast count equal to or greater than 5%. Non-relapse mortality (NRM) was defined as death from any cause other than disease progression. Acute graft versus host disease (GVHD) was clinically graded as 0 to IV based on standard criteria 40; chronic GVHD was classified as none, limited, or extensive 41. Acute GVHD, which persisted or progressed after day 100, was also scored as chronic GVHD in this study. Counts of patients by baseline characteristics were tabulated for this retrospective cohort. Patient characteristics were compared between patients with and without pre-transplant MRD using the chi-square tests, with two exceptions. If there were expected cell counts of less than 5, then the exact test p-value was used 42. For variables with a natural ordering in the categories, the exact p-value for the Jonckheere-Terpstra (J-T) test 43 was used to accommodate the ordering and small sample sizes. Overall survival was defined as the time from transplant until death. Patients alive at last contact were censored for OS on the date of last contact. Progression-free survival was the time from transplant until death or progression, whichever came first. Patients alive and free of progression at the last follow-up for progression were censored for PFS on the date of last follow-up. Additionally, the time to development of post-transplant MRD (TTMRD) was defined as the time from transplant to the subsequent development of MRD. Patients who never developed MRD were censored for TTMRD at the date of last biopsy. Post-MRD measures of OS and PFS were measured and censored similarly among patients who developed MRD, but with the starting date as MRD detection instead of transplant. Kaplan-Meier estimates 44 were calculated for OS and PFS overall and for patient characteristics at transplant. The relationship between each of these characteristics and measures were tested with log-rank tests. Post-transplant MRD and extensive chronic GVHD were tested as time-varying covariates. All measures were tested in a proportional hazards regression model 45 using time-varying covariates as needed. All variables except allotype were entered into the model initially. A backwards selection model was performed requiring MRD to remain in the model, but then sequentially eliminating the least significant variable until all remaining variables had a p-value of 0.05 or less. The time to MRD was estimated by the Nelson-Aalen 46,47 cumulative hazard function. Chi-square and J-T tests were performed in Cytel Studio 8 (Cytel Inc., Cambridge, MA). Time-to-event analyses were performed in SAS 9.2 (SAS Institute Inc, Cary, NC) and figures were produced in STATA 12.1 (StatCorp LP, College Station, TX).
RESULTS
Patient and treatment characteristics
Minimal residual disease was detected in 32 patients at time of HSCT (21%). Patient demographics and baseline disease characteristics are listed in Table 1 based on association with MRD. There were 91 (61%) men and 58 (39%) women, with a median age 36 years (range, 18-70 years). One hundred twenty-nine (87%) had B-lymphoblastic leukemia and 20 (13%) patients had T-lymphoblastic leukemia/lymphoma. Among 134 patients with available cytogenetic data at diagnosis, 80 (60%) were considered high risk defined by the presence of the t(9;22), t(4;11), hypodiploid or complex cytogenetics, 2 patients (1%) were classified as good risk by the presence of hyperdiploidy, and the remaining 52 patients (39%) were intermediate risk. Specifically, 30 patients had the t(9;22) translocation. At time of transplant, all patients were in complete remission, including 72 (48%) in first CR (CR1), 70 (47%) in CR2, and 7 (5%) in CR3. All patients received HLA-matched transplants with either related (n=84) or unrelated donors (n=65). The stem cell source was either bone marrow (n=47) or peripheral blood (n=102). All patients received myeloablative conditioning with TBI (n=39), or without (n=110). Patients were uniformly treated with tacrolimus and mini-dose methotrexate for GVHD prophylaxis. Patient age, disease status at time of HSCT, and cytogenetic risk were significantly associated with MRD status. There was a trend for the association of sex with MRD status.
Table 1.
Patient Characteristics by Pre-Transplant MRD Status
| Pre-Transplant MRD | |||
|---|---|---|---|
| No | Yes | ||
| Characteristic | N (%) | N (%) | P-value |
| All Patients | 117 (79) | 32 (21) | |
| Age | 0.05 | ||
| <40 years | 65 (73) | 24 (27) | |
| ≥40 years | 52 (87) | 8 (13) | |
| Sex | 0.31 | ||
| Male | 69 (76) | 22 (24) | |
| Female | 48 (83) | 10 (17) | |
| Disease Status* | 0.003 | ||
| 1st CR/CRi | 65 (90) | 7 (10) | |
| 2nd CR/CRi | 46 (66) | 24 (34) | |
| 3rd CR/CRi | 6 (86) | 1 (14) | |
| Cytogenetic Risk* | 0.02 | ||
| Good | 2 (100) | 0 (0) | |
| Intermediate | 35 (67) | 17 (33) | |
| High | 69 (86) | 11 (14) | |
| Unknown** | 11 (73) | 4 (27) | |
| Phenotype | >0.99 | ||
| T cell | 16 (80) | 4 (20) | |
| B cell | 101 (78) | 28 (22) | |
| Conditioning Regimen | 0.23 | ||
| TBIMAC | 28 (72) | 11 (28) | |
| nonTBIMAC | 89 (81) | 21 (19) | |
| Allotype | 0.11 | ||
| Matched Related | 62 (74) | 22 (26) | |
| Matched Unrelated | 55 (85) | 10 (15) | |
| Cell Type | 0.37 | ||
| PB (HPC-A) | 78 (76) | 24 (24) | |
| BM (HPC-M) | 39 (83) | 8 (17) | |
Due to the natural ordering of these variables, they were tested with the JT test.
Patients with Unknown Cytogenetic Risk were not included in the analysis
Prognostic significance of MRD detected at time of HSCT
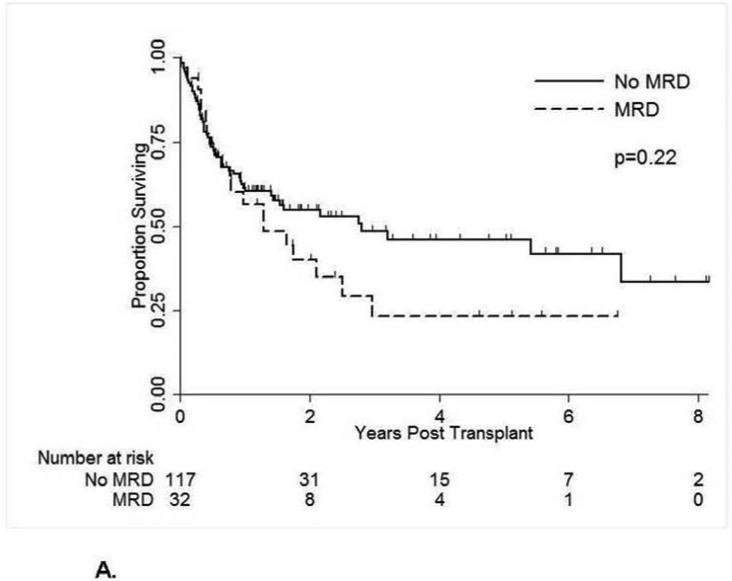
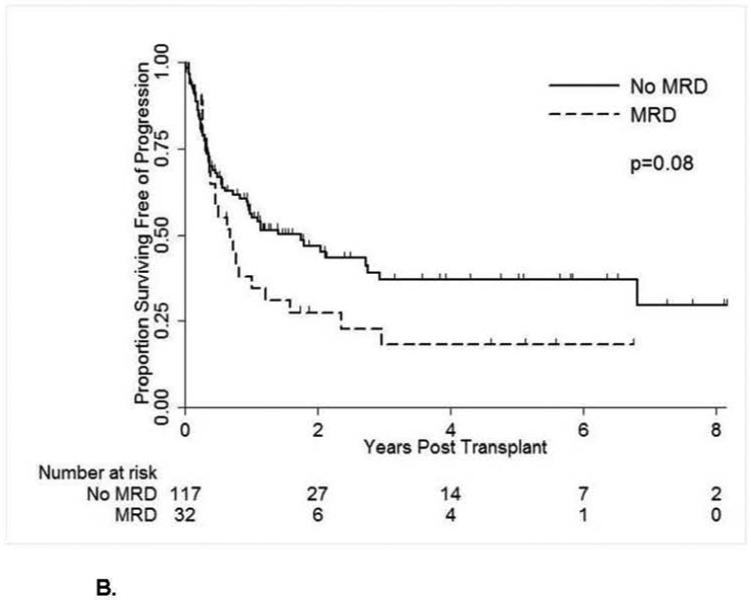
A total of 73 patients died with a 2-year OS estimate of 51%. Patients without pre-transplant MRD had a longer OS, but not significantly so, with 55% vs. 40% alive at 2 years (Table 2, Figure 1A). A total of 84 patients relapsed or died for a progression-free survival of 42% at 2 years, with a trend for better PFS in patients without MRD at time of HSCT, 47% versus 28% (p=.08) (Figure 1B). In multivariate analysis, the absence of pre-transplant MRD was associated with better PFS (hazard ratio (HR) = 0.62) nearing significance (p=.07) (Table 3). When MRD was analyzed as high (>0.1%) vs low (≤ 0.1% and ≥ 0.01%) levels, the higher levels of MRD were associated with a higher rate of relapse, with 2-year PFS 21% vs. 34%, but not statistically significant, p=0.19 (Table 2). Additionally, older age and disease stage greater than CR1 were associated with significantly worse OS and PFS in multivariate analyses (Table 3). Furthermore, unrelated allotype (p=0.02) and peripheral blood grafts (p=0.04) were associated with worse OS but not PFS in multivariate analyses (Table 3).
Table 2.
Disease and Survival Outcomes by Pre-Transplant Characteristics (Univariate)
| OS |
PFS |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | Deaths | 2-year estimate (SE) | P-value | Events | 2-year estimate (SE) | P-value |
| All Patients | 149 | 73 | 51% (5%) | 84 | 42% (5%) | ||
| MRD Pre-Transplant | 0.22 | 0.08 | |||||
| No | 117 | 53 | 55% (5%) | 60 | 47% (5%) | ||
| Yes | 32 | 20 | 40% (10%) | OS | 24 | 28% (8%) | PFS |
| MRD Pre-Transplant | 0.46 | 0.19 | |||||
| No (0) | 117 | 53 | 55% (5%) | 60 | 47% (5%) | ||
| Low (≤0.1) | 17 | 10 | 38% (13%) | 11 | 34% (12%) | ||
| High (>0.1) | 15 | 10 | 44% (14%) | 13 | 21% (11%) | ||
| Age | 0.02 | 0.05 | |||||
| <40 years | 89 | 40 | 59% (6%) | 47 | 47% (6%) | ||
| ≥40 years | 60 | 33 | 38% (8%) | 37 | 33% (7%) | ||
| Sex | 0.05 | 0.16 | |||||
| Male | 91 | 50 | 45% (6%) | 54 | 37% (6%) | ||
| Female | 58 | 23 | 61% (7%) | 30 | 49% (7%) | ||
| Disease Status | 0.08 | 0.07 | |||||
| 1st CR/CRi | 72 | 29 | 63% (6%) | 36 | 51% (7%) | ||
| Later CR/CRi | 77 | 44 | 41% (6%) | 48 | 34% (6%) | ||
| Cytogenetic Risk | 0.98 | 0.71 | |||||
| High | 80 | 39 | 55% (6%) | 47 | 44% (6%) | ||
| Intermediate/Good | 54 | 26 | 48% (8%) | 29 | 39% (7%) | ||
| Phenotype | 0.70 | 0.67 | |||||
| T cell | 20 | 10 | 31% (13%) | 11 | 33% (12%) | ||
| B cell | 129 | 63 | 54% (5%) | 73 | 44% (5%) | ||
| Conditioning Regimen |
0.50 | 0.22 | |||||
| TBI | 39 | 21 | 60% (8%) | 22 | 52% (8%) | ||
| nonTBI | 110 | 52 | 47% (6%) | 62 | 37% (6%) | ||
| Allotype | 0.21 | 0.57 | |||||
| Matched Related | 84 | 38 | 53% (6%) | 47 | 43% (6%) | ||
| Matched Unrelated | 65 | 35 | 49% (7%) | 37 | 42% (7%) | ||
| Cell Type | 0.19 | 0.11 | |||||
| PB (HPC-A) | 102 | 53 | 48% (5%) | 62 | 40% (5%) | ||
| BM (HPC-M) | 47 | 20 | 59% (8%) | 22 | 48% (8%) | ||
Figure 1A.
Overall Survival by Pre-Transplant Minimal Residual Disease Status
Figure 1B.
Progression-Free Survival by Pre-Transplant Minimal Residual Disease Status
Table 3.
Survival and Disease Outcomes based Pre-Transplant Characteristics
| (73 Events) | (84 Events) | |||
|---|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| MRD Pre-Transplant (No: Yes) | 0.66 (0.37, 1.15) | 0.14 | 0.62 (0.37, 1.04) | 0.07 |
| Age (<40:≥40) | 0.49 (0.30, 0.82) | 0.01 | 0.50 (0.31, 0.81) | 0.004 |
| Disease Status (1st: Later CR/CRi) | 0.60 (0.36, 0.99) | 0.05 | 0.63 (0.397, 1.00) | 0.05 |
| Allotype (Related: Unrelated) | 0.54 (0.32, 0.92) | 0.02 | NI | |
| Cell Type (PB: BM) | 1.9 (1.03, 3.34) | 0.04 | NI | |
NI =Not Included, indicates that the variable was not included in the final model after the selection procedure was finished. Every model was required to include MRD. The remaining variables in the full model were age, sex, disease status, histology, conditioning regimen, allotype, and cell type. Only variables that were significantly associated with at least one outcome after model selection are listed in the table.
Prognostic significance of MRD detected after HSCT
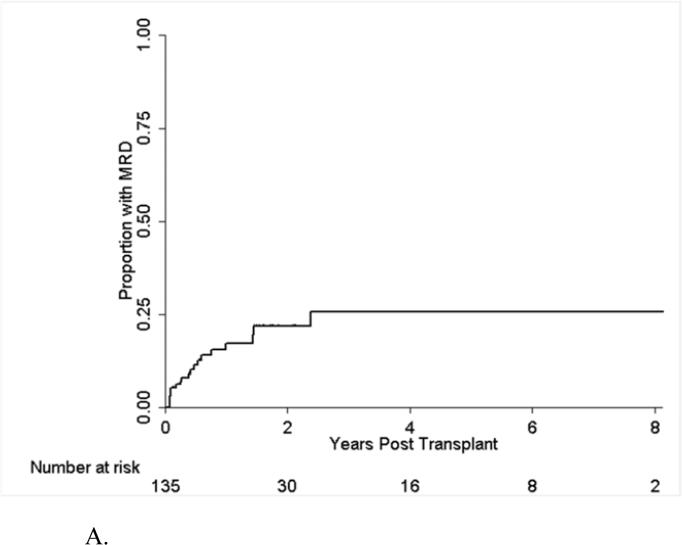
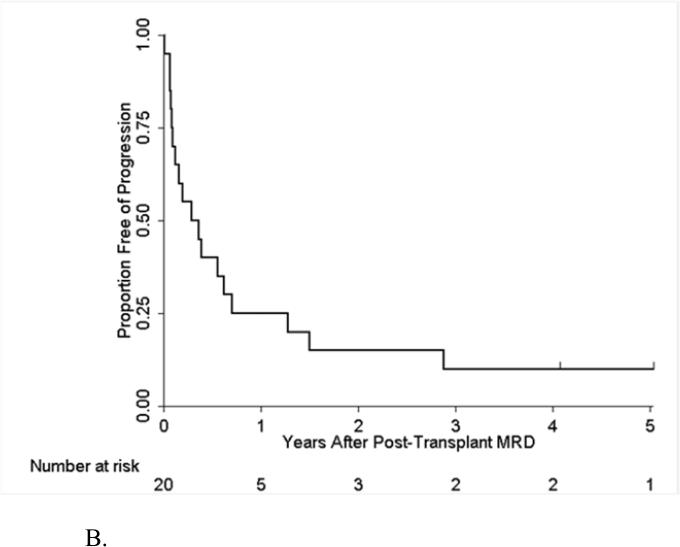
One hundred thirty-five patients were monitored for post-HSCT MRD by FCI starting at the first month after transplant; 20 of them became positive for MRD with an overall post-transplant MRD rate of 25% (Figure 2A). Thirty of the 135 patients were still free of MRD at 2 years. The presence of MRD post HSCT was associated with significantly worse PFS and OS in univariate and multivariate analyses (Table 4). Among the 20 MRD positive patients, 18 (90%) developed overt hematological relapse. For 7 patients with MRD detected at 30 days post-HSCT, 6 developed hematological relapse (86%); whereas of 13 patients with MRD detected more than 1 month after HSCT, 12 (92%) developed hematological relapse. The median time between detection of MRD post HSCT and overt hematological relapse was 3.8 months (95% CI: 0.9, 8.4 months) (Figure 2B). Not all patients with disease progression had preceding detectable MRD by FCI. Among 32 patients with hematological relapse, 13 (41%) patients did not have MRD in the preceding MRD assessment performed within 3 months prior to the overt relapse. The type of transplant conditioning (with or without TBI) did not impact progression in this group (data not shown). Furthermore, five patients with Ph+ ALL received post transplant TKI therapy, and again there was no impact on progression.
Figure 2A.
Time to MRD after Transplant.
Table 4.
Survival and Disease Outcomes by Characteristics and Post-Transplant MRD and GVHD for 135 Patients with Post-Transplant MRD Measurements
| OS (60 Events) | PFS (70 Events) | |||
|---|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| Univariate MRD Post-Transplant* (Yes: No) | 3.3 (1.8, 5.8) | <0.001 | 3.6 (2.1, 6.3) | <0.001 |
| Univariate cGVHD Post-Transplant* (Yes: No) | 1.8 (0.9, 3.6) | 0.09 | 1.3 (0.7, 2.4) | 0.46 |
| Multivariate Analyses | ||||
| MRD Post-Transplant* (Yes: No) | 3.5 (2.0, 6.2) | <0.001 | 4.4 (2.5, 7.8) | <0.001 |
| Disease Status (1st Cr/CRi: not 1st CR/CRi) | NI | 2.1 (1.2, 3.7) | 0.01 | |
| Phenotype (B cell: T cell) | 0.5 (0.3, 1.0) | 0.05 | NI | |
| Conditioning Regimen (nonTBI:TBI) | 2.1 (1.1, 4.1) | 0.03 | NI | |
NI =Not Included, indicates that the variable was not included in the final model after the selection procedure was finished. Every model was required to include post-transplant MRD. The remaining variables in the full model were pre-transplant MRD age, disease status, histology, conditioning regimen, cell type, AGVHD, and CGVHD. Only variables that were significantly associated with at least one outcome are listed in the table.
MRD Post-Transplant and cGVHD were included as time-varying covariates, so the group size changed over time. In all, 20 patients had MRD detected after transplant and 21 had extensive cGVHD.
Figure 2B.
Time to Progression after Post-Transplant MRD among Patients with MRD
DISCUSSION
To our knowledge, this is one of the largest studies on ALL MRD in adults using a uniform detection method at the time of HSCT and following transplant. We demonstrated that the presence of MRD detected by FCI at time of HSCT is nearly a significant risk factor for post-HSCT relapse in univariate and multivariate analyses (Tables 2 and Table 3, Figure 1A). We were unable to show significance likely due to the relatively small sample size. Furthermore, we demonstrated that patients with MRD at time of transplant were more likely to be younger, have intermediate-risk cytogenetics, and have disease beyond first remission (Table 1). The associations of MRD with age and cytogenetic classification may reflect our practice pattern of more commonly transplanting intermediate risk patients, i.e. younger patients with non-high risk cytogenetics, only if they have persistent MRD. The association of MRD and advanced disease stage follows biology. Finally, MRD detected after HSCT was highly predictive for overt hematological relapse, with a median latency interval of approximately 3.8 months (Table 4, Figure 2).
The significance of MRD detected at time of transplant raises an important question as to whether we can derive benefit by further treating the patient to lower the MRD burden prior to transplant. The dose effect of MRD burden at time of transplant on risk of relapse has been well documented in children. Leung and colleagues recently reported a higher rate of relapse (40% at 5-year) in patients with higher MRD burden (≥0.1%) detected by FCI, as compared to 16% in patients with a MRD burden between 0.01% and 0.1%48. Similar trends have been shown in prior pediatric studies by PCR methods, where patients with higher levels (≥ 0.1%) of pre-HSCT MRD had inferior 5-year event-free-survival (EFS) (21%) than patients with lower level (< 0.1%) MRD (41%) or without MRD (75%) 26-28, 49. In this study, we showed similar findings for progression in adults with ALL: the subset of patients with MRD ≥0.1% had the shortest PFS (21% at 2 years) as compared with the subset of patients with MRD <0.1% (34% at 2 years) and without MRD (47% at 2 years); we did not find significance, p=0.19 (Table 2). However, we were not able to study this important question in detail due, or find significance, possibly due to small patient numbers. This is an important clinical question since treating patients more intensely to decrease disease burden is associated with more treatment-related toxicity, and the GVL effect may be able to control a certain disease “level”. However, this level has not been defined in the current literature, and the differential effect of MRD burden on disease relapse likely contributes to the discrepancies noted in studies, with some reporting a higher risk for relapse 21, 29 and others not 23. Small patient numbers also precluded us from studying the impact of MRD by disease status. Due to the small sample size, we grouped patients with remissions beyond CR1 together despite the fact that biologically they may be more resistant to treatment, and thus the presence of MRD in the more advanced disease group may have different impact.
In this study, we also found that MRD detected by FCI after transplant was closely associated with overt hematological relapse. The risk was strongly significant in both univariate (hazard ratio =3.6, p < .001) and multivariate (hazard ratio =4.4, p < .001) analyses (table 4). These findings suggest that post-HSCT MRD is an important predictor for overt hematological relapse; MRD monitoring after HSCT at 2-3 month intervals may detect as many as 60% of patients with emerging hematological relapse, which may provide a window of opportunity for early intervention. Of note, in our study and the study by Zhao et al, approximately 40% of post-transplant relapses had no preceding MRD detectedwithin 3 months before diagnosis of relapse. Overt hematological relapse without preceding MRD may be attributed to rapid disease progression or low detection sensitivity due to patchy distribution of the residual disease. Hemodilution of the bone marrow aspirate sample also can be a contributing factor, especially if the latter portion of the aspirate specimen is collected for flow cytometric studies.
CONCLUSION
In conclusion, we noted an association for MRD, detected by FCI before and after HSCT, and subsequent hematological relapse. Our findings are confined to the level of sensitivity of our FCI method, and small patient numbers precluded more detailed study of MRD burden and relapse risk. Still, our observations, which are in accordance with the bulk of the MRD literature, have important clinical ramifications. For patients with Ph+ ALL, the presence of MRD would warrant use of TKIs; for Ph negative patients novel interventions are needed. Additionally, the burden of MRD may have a differential impact on relapse, and may be a useful guide for the intensification of treatment prior to transplant, and the intensification of the transplant preparative regimen. Finally, the prognostic utility of MRD after HSCT is highly specific, but its sensitivity is limited since approximately 40-50% of patients can develop relapse without MRD in the preceding 3 months. These findings need to be further investigated in large, prospective studies.
Clinical Practice Points.
This study corroborates the existing literature on the prognostic importance of minimal residual disease (MRD) in patients undergoing therapy for acute lymphoblastic leukemia (ALL). The presence of MRD at time of transplant indicates the need for additional intervention in efforts to minimize the risk for relapse.
Acknowledgment
The authors thank the clinical and laboratory staff for the care of the patients and laboratory processing of patient samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: All the authors declare no conflict of interest.
Authorship Contributions: Y.Z. designed the research, interpreted data, and wrote the manuscript; R. S. performed statistical analysis and wrote the manuscript; J.L.J. designed research and wrote the manuscript; S. A. W. collected data; G.R. collected data; M.D. collected data; E.J.S. collected data; U.P. collected data; S.C. collected data; A.A. collected data; M.Q. collected data; C.H. collected data; S.O. collected data; D.T. collected data; H.K. collected data; L.J.M. designed research; R.E.C. designed research; P.K. designed research, interpreted data and wrote the manuscript.
Reference
- 1.Dinsmore R, Kirkpatrick D, Flomenberg N, Gulati S, Kapoor N, Shank B, et al. Allogeneic bone marrow transplantation for patients with acute lymphoblastic leukemia. Blood. 1983;62(2):381–8. [PubMed] [Google Scholar]
- 2.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827–33. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 3.Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489–96. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett AJ, Horowitz MM, Ash RC, Atkinson K, Gale RP, Goldman JM, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992;79(11):3067–70. [PubMed] [Google Scholar]
- 5.Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107(12):4961–7. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uderzo C, Valsecchi MG, Bacigalupo A, Meloni G, Messina C, Polchi P, et al. Treatment of childhood acute lymphoblastic leukemia in second remission with allogeneic bone marrow transplantation and chemotherapy: ten-year experience of the Italian Bone Marrow Transplantation Group and the Italian Pediatric Hematology Oncology Association. J Clin Oncol. 1995;13(2):352–8. doi: 10.1200/JCO.1995.13.2.352. [DOI] [PubMed] [Google Scholar]
- 7.Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–8. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingard JR, Piantadosi S, Santos GW, Saral R, Vriesendorp HM, Yeager AM, et al. Allogeneic bone marrow transplantation for patients with high-risk acute lymphoblastic leukemia. J Clin Oncol. 1990;8(5):820–30. doi: 10.1200/JCO.1990.8.5.820. [DOI] [PubMed] [Google Scholar]
- 9.Malempati S, Gaynon PS, Sather H, La MK, Stork LC. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J Clin Oncol 2007. 25(36):5800–7. doi: 10.1200/JCO.2007.10.7508. [DOI] [PubMed] [Google Scholar]
- 10.Barrett AJ, Horowitz MM, Gale RP, Biggs JC, Camitta BM, Dicke KA, et al. Marrow transplantation for acute lymphoblastic leukemia: factors affecting relapse and survival. Blood. 1989;74(2):862–71. [PubMed] [Google Scholar]
- 11.Woolfrey AE, Anasetti C, Storer B, Doney K, Milner LA, Sievers EL, et al. Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood. 2002;99(6):2002–8. doi: 10.1182/blood.v99.6.2002. [DOI] [PubMed] [Google Scholar]
- 12.Attarbaschi A, Mann G, Panzer-Grumayer R, Rottgers S, Steiner M, Konig M, et al. Minimal residual disease values discriminate between low and high relapse risk in children with B-cell precursor acute lymphoblastic leukemia and an intrachromosomal amplification of chromosome 21: the Austrian and German acute lymphoblastic leukemia Berlin-Frankfurt-Munster (ALL-BFM) trials. J Clin Oncol. 2008;26(18):3046–50. doi: 10.1200/JCO.2008.16.1117. [DOI] [PubMed] [Google Scholar]
- 13.Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168–74. doi: 10.1200/JCO.2008.20.8934. [DOI] [PubMed] [Google Scholar]
- 14.Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood. 2010;115(23):4657–63. doi: 10.1182/blood-2009-11-253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010. 115(16):3206–14. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 16.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood. 2008;111(12):5477–85. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Goldwasser MA, Li A, Dahlberg SE, Neuberg D, Wang H, et al. Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood. 2007;110(5):1607–11. doi: 10.1182/blood-2006-09-045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raff T, Gokbuget N, Luschen S, Reutzel R, Ritgen M, Irmer S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910–5. doi: 10.1182/blood-2006-07-037093. [DOI] [PubMed] [Google Scholar]
- 19.Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116–23. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 20.Panzer-Grumayer ER, Schneider M, Panzer S, Fasching K, Gadner H. Rapid molecular response during early induction chemotherapy predicts a good outcome in childhood acute lymphoblastic leukemia. Blood. 2000;95(3):790–4. [PubMed] [Google Scholar]
- 21.Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153–62. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- 22.Mortuza FY, Papaioannou M, Moreira IM, Coyle LA, Gameiro P, Gandini D, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20(4):1094–104. doi: 10.1200/JCO.2002.20.4.1094. [DOI] [PubMed] [Google Scholar]
- 23.Patel B, Rai L, Buck G, Richards SM, Mortuza Y, Mitchell W, et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol. 2010;148(1):80–9. doi: 10.1111/j.1365-2141.2009.07941.x. [DOI] [PubMed] [Google Scholar]
- 24.Goulden N, Bader P, Van Der Velden V, Moppett J, Schilham M, Masden HO, et al. Minimal residual disease prior to stem cell transplant for childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;122(1):24–9. doi: 10.1046/j.1365-2141.2003.04394.x. [DOI] [PubMed] [Google Scholar]
- 25.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–84. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 26.Knechtli CJ, Goulden NJ, Hancock JP, Grandage VL, Harris EL, Garland RJ, et al. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92(11):4072–9. [PubMed] [Google Scholar]
- 27.Krejci O, van der Velden VH, Bader P, Kreyenberg H, Goulden N, Hancock J, et al. Level of minimal residual disease prior to haematopoietic stem cell transplantation predicts prognosis in paediatric patients with acute lymphoblastic leukaemia: a report of the Pre-BMT MRD Study Group. Bone Marrow Transplant. 2003;32(8):849–51. doi: 10.1038/sj.bmt.1704241. [DOI] [PubMed] [Google Scholar]
- 28.Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16(9):1668–72. doi: 10.1038/sj.leu.2402552. [DOI] [PubMed] [Google Scholar]
- 29.Spinelli O, Peruta B, Tosi M, Guerini V, Salvi A, Zanotti MC, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92(5):612–8. doi: 10.3324/haematol.10965. [DOI] [PubMed] [Google Scholar]
- 30.Uzunel M, Jaksch M, Mattsson J, Ringden O. Minimal residual disease detection after allogeneic stem cell transplantation is correlated to relapse in patients with acute lymphoblastic leukaemia. Br J Haematol. 2003;122(5):788–94. doi: 10.1046/j.1365-2141.2003.04495.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhao XS, Liu YR, Zhu HH, Xu LP, Liu DH, Liu KY, et al. Monitoring MRD with flow cytometry: an effective method to predict relapse for ALL patients after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2011;91(2):183–192. doi: 10.1007/s00277-011-1285-1. [DOI] [PubMed] [Google Scholar]
- 32.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(3):367–9. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vriesendorp HM, Chu H, Ochran TG, Besa PC, Champlin RE. Radiobiology of total body radiation. Bone Marrow Transplant. 1994;14(Suppl 4):S4–8. [PubMed] [Google Scholar]
- 34.Gopal R, Ha CS, Tucker SL, Khouri IF, Giralt SA, Gajewski JL, et al. Comparison of two total body irradiation fractionation regimens with respect to acute and late pulmonary toxicity. Cancer. 2001;92(7):1949–58. doi: 10.1002/1097-0142(20011001)92:7<1949::aid-cncr1714>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Kebriaei P, Saliba RM, Ma C, Ippoliti C, Couriel DR, de Lima M, et al. Allogeneic hematopoietic stem cell transplantation after rituximab-containing myeloablative preparative regimen for acute lymphoblastic leukemia. Bone Marrow Transplant. 2006;38(3):203–9. doi: 10.1038/sj.bmt.1705425. [DOI] [PubMed] [Google Scholar]
- 36.Kebriaei P, Madden T, Wang X, Thall PF, Ledesma C, de Lima M, et al. Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transplant. 2013;48(1):26–31. doi: 10.1038/bmt.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kebriaei P, Basset R, Ledesma C, Ciurea S, Parmar S, Shpall EJ, et al. Clofarabine combined with busulfan provides excellent disease control in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(12):1819–26. doi: 10.1016/j.bbmt.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravandi F, Jorgensen J, Thomas DA, O'Brien S, Garris R, Faderl S, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome -positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortes J, Talpaz M, O'Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11(9):3425–32. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 40.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 41.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57(2):267–76. [PubMed] [Google Scholar]
- 42.Mehta C, Patel N. StatXact4 for Windows. 2000:605–610. [Google Scholar]
- 43.Mehta C, Patel N. StatXact4 for Windows. 2000:384–388. [Google Scholar]
- 44.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 45.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 46.Nelson W. Theory and applications of hazard plotting for censored failure data. Technometrics. 1972;14:945–966. [Google Scholar]
- 47.Aalen OO. Nonparametric inference for a family of counting processes. Annals of Statistics. 1978;6:701–726. [Google Scholar]
- 48.Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012 doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Velden VH, Joosten SA, Willemse MJ, van Wering ER, Lankester AW, van Dongen JJ, et al. Real-time quantitative PCR for detection of minimal residual disease before allogeneic stem cell transplantation predicts outcome in children with acute lymphoblastic leukemia. Leukemia. 2001;15(9):1485–7. doi: 10.1038/sj.leu.2402198. [DOI] [PubMed] [Google Scholar]