Abstract
Background
The malignant potential of intraductal mucinous neoplasm of the pancreas (IPMN) is associated closely with main pancreatic duct (MPD) involvement. Because mixed-type IPMN is thought to have the same malignant potential as that of main-duct (MD)-IPMN, resection is recommended; however, the biological nature of mixed-type IPMN with only minimal involvement of MPD (min-mix-IPMN) may be different.
Methods
A prospective database of 404 resected IPMNs was re-reviewed to subclassify mixed-type IPMNs. We defined min-mix-IPMN as absence of gross abnormalities (except for dilatation) of MPD and noncircumferential microscopic involvement of MPD limited to few sections.
Results
We identified 46 min-mix-IPMNs, 163 IPMNs with extensive involvement of MPD (ex-mix-IPMN), 175 branch-duct (BD)-IPMNs, and 20 MD-IPMNs. The majority of min-mix-IPMNs were found incidentally and increased cyst size on surveillance was the leading operative indication. The median diameter of MPD was 2 mm in min-mix-IPMN versus 9 mm in ex-mix-IPMN (P < .0001), and cysts ≥10 mm were present in 62% of ex-mix-IPMNs versus 93% of min-mix-IPMNs (P < .0001). Most importantly, the vast majority of min-mix-IPMNs exhibited gastric-type epithelium, similar to BD-IPMNs, whereas intestinal-type epithelium was present in half of ex-mix-IPMNs, similar to MD-IPMNs. The prevalence of high-grade lesions was less in min-mix-IPMN than ex-mix-IPMN (P < .0001). These differences were reflected in better disease-specific outcomes of min-mix-IPMN compared with ex-mix-IPMN (P = .046).
Conclusion
Min-mix-IPMN often presents with no MPD dilation and is an incidental finding by microscopic examination. min-mix-IPMN shares the pathologic features and less aggressive biology with BD-IPMN. We propose that min-mix-IPMN be categorized differently than ex-mix-IPMN.
In the last 2 decades, intraductal mucinous neoplasm of the pancreas (IPMN) has attracted tremendous attention in the field of pancreatology. Since its first description,1 multiple series with increasing numbers of cases revealed IPMN to be a disease with a heterogeneous nature.2-6 Without doubt, all IPMNs harbor a risk of malignant transformation, following an adenoma carcinoma sequence, similar to the other gastrointestinal malignancies,7 but each type of IPMN lesion carries a different degree of malignant potential, and several clinicopathologic characteristics including macroscopic types (the differential involvement of the pancreatic duct system) seem to predict the risk of malignant transformation.2,5,8 Large series of resected IPMNs have shown a high prevalence of high-grade dysplasia and invasive cancer (mean, 61.6%; range, 36–100%) in lesions involving predominantly the main pancreatic duct (MPD; main-duct type IPMN [MD-IPMN]), whereas the prevalence of high-grade dysplasia and invasive cancer is much less (mean, 25.5%; range, 6.3–46.5%) in those limited to side branches (branch-duct type IPMN [BD-IPMN]).4,9-11 Lesions involving both main and side branches (mixed-type IPMN) are thought to have a similar biology to MD-IPMNs.2,10,12 Unfortunately, however, the criteria used to differentiate MD-IPMN versus BD-IPMN versus mixed-type IPMN were not uniform in these studies; the differentiation was based solely on the results of preoperative imaging studies in some, and the differentiation was based on the pathology evaluation of resected specimens in the others. Nevertheless, the international guidelines recommend resection of all IPMNs with MPD involvement depicted by preoperative imaging studies.13,14
Several recent studies indicate that the macroscopic types of IPMN and their biologic behavior are related closely to epithelial subtypes of their (intraductal) papillary components, which can be classified into gastric, intestinal, oncocytic, and pancreatobiliary types.15-17 The gastric-type lesions found in the vast majority of BD-IPMNs exhibit commonly low- to intermediate-grade dysplasia and progress infrequently into cancer, whereas the intestinal-type lesions frequently seen in MD-IPMNs are typically classified as high-grade dysplasia with or without invasive components. Of note, the gastric-type epithelium often coexists with the other epithelial types and may represent a precursor to the others.18 There is no evidence that branch-duct lesions extend into MPD over time, forming mixed-type IPMN.
In our experience, there seem to be 2 types of mixed-type IPMN based on pathology evaluation–one with extensive involvement of the main duct (ex-mix-IPMN) and the other with microscopic (minimal) involvement of the main duct (min-mix-IPMN). The latter may be difficult to distinguish from BD-IPMN preoperatively; therefore, it is of clinical importance to determine whether min-mix-IPMN harbors the same risk of malignant transformation as ex-mix-IPMN. To our knowledge, no studies have analyzed mixed-type IPMNs as a heterogeneous group with various degrees of MPD involvement. The purpose of this study was to determine the biologic nature of min-mix-IPMN and that of ex-mix-IPMN by evaluating and comparing their clinicopathologic characteristics and prognosis between each other and with those of BD-IPMN and MD-IPMN in a large cohort of patients.
MATERIALS AND METHODS
Study patients
The study protocol was approved by the Massachusetts General Hospital Internal Review Board. The prospective IPMN database of Massachusetts General Hospital Department of Surgery was queried to identify patients with MD-IPMN, mixed-type-IPMN and BD-IPMN, who underwent operation between January 1993 and December 2012 (n = 404). Patient demographic, surgical, radiologic, and pathologic characteristics were recorded.
Imaging studies
All patients underwent preoperative computed tomography and/or magnetic resonance imaging, the latter including magnetic resonance cholangiopancreatography. In several cases additional information by endoscopic ultra-sonography with or without fine needle aspiration and/or endoscopic retrograde cholangiopancreatography was available. A MPD diameter of ≥5 mm was recorded as duct dilation in this study.
Histopathologic evaluation
In each case, the entire cystic lesion(s) as well as all the gross abnormalities seen in the MPD were submitted for microscopic examination. In addition, multiple sections (average, 2; range, 1–5) of the grossly unremarkable main duct including the segment immediately associated with the cystic lesion were microscopically evaluated. Histologic assessment was performed according to the World Health Organization criteria for IPMN and each case was graded as low-, intermediate-, or high-grade dysplasia, or invasive carcinoma. The intraductal components were classified into 4 distinct epithelial subtypes–gastric, intestinal, pancreatobiliary, and oncocytic–on the basis of their epithelial morphology on routine hematoxylin and eosin staining, and, when available, immunoreactivity against mucin glycoproteins according to criteria described previously.16,19 In cases exhibiting heterogeneous epithelium, the subtype was determined on the basis of the most prevalent epithelium of the highest grade. The histology of invasive components, if present, were classified as tubular, colloid, or oncocytic carcinoma.15 For the purpose of this study, the type of duct involvement by IPMN was determined by microscopic examination irrespective of the results of clinical imaging studies and/or macroscopic appearance. The microscopic involvement of MPD by mucinous epithelium (of any grade) was required for the pathologic diagnosis of mixed-type or MD-IPMN with or without the involvement of branch ducts, whereas mucinous epithelium was confined to side branches in BD-IPMN.
All identified mixed-type IPMNs were re-reviewed by a GI pathologist with a special interest in the field of pancreas (M.M.-K.) and were classified as min-mix-IPMN and ex-mix-IPMN based on the extent of MPD involvement. Minimal involvement was defined as absence of gross abnormality (except for MPD dilation) and non-circumferential microscopic involvement of MPD limited to 1 or a few histologic sections (Fig 1). Mixed-type IPMNs not fulfilling these requirements were classified as ex-mix-IPMN. In all min-mix-IPMNs, the histologic grade and epithelial subtype were assessed for the main and branch-duct components separately.
Fig 1.
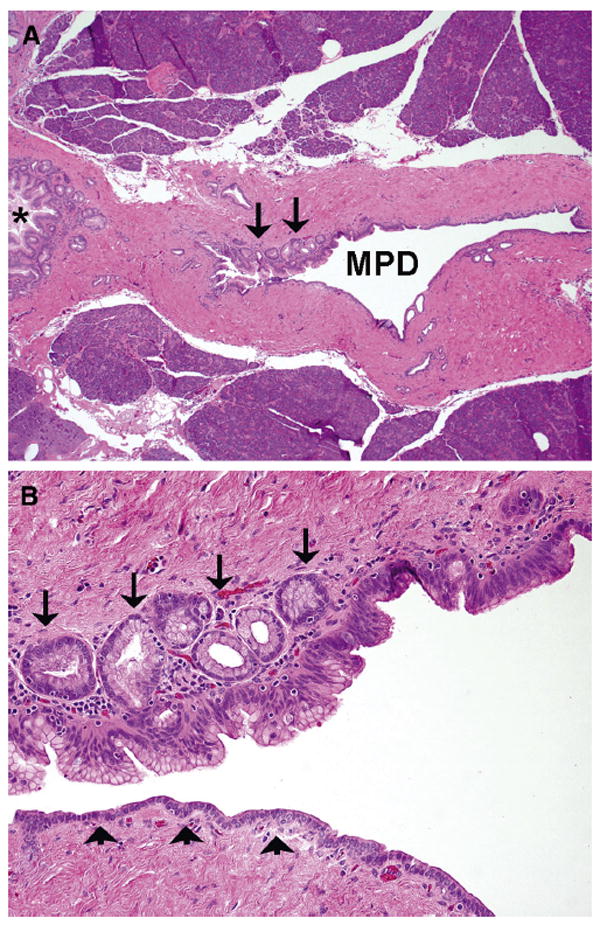
A, The main pancreatic duct (MPD) is focally involved by mucinous epithelium (arrows), whereas a large branch duct (*) is involved circumferentially. B, A greater magnification demonstrates focal involvement of MPD by the gastric-type epithelium of low- to intermediate-grade dysplasia. The arrows indicate pancreatic duct glands undermining the luminal mucinous epithelium and arrowheads, normal pancreatic epithelium without cytoplasmic mucin.
Follow-up
The individual last follow-up status was evaluated by electronic medical records and the social security death index. Additional follow-up evaluation included clinical examination and/or imaging studies such as computed tomography or magnetic resonance imaging. All patients were interviewed biannually by a study nurse if no more clinical follow-up was indicated. IPMN recurrence was defined as appearance of a new cystic lesion, likely to be an IPMN (duct communication, multi-focality), on computed tomography or magnetic resonance imaging follow-up imaging, or newly identified main-duct abnormalities. Survival time was defined from the date of death. Follow-up time was assessed from the date of operation to the last follow-up date, no later than December 31, 2012.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. Continuous variables are expressed by median and range, and compared by the Mann–Whitney U test or a 2-sample Student t test. For survival analysis, the Kaplan–Meier method was used to assess survival time distribution, and log-rank test was applied. All tests were 2-sided. SPSS 18 for Mac OsX (SPSS Inc., Chicago, IL), and GraphPad Prism 6 were used for analysis.
RESULTS
Clinical characteristics of patients with mixed-type IPMN
Among 404 resected IPMNs, 209 (52%) were histopathologically classified as mixed-type IPMN. Of those, 46 were subclassified as min-mix-IPMNs and formed our study group. The remaining 163 mixed-type IPMNs that were classified as ex-mix-IPMNs and 175 BD-IPMNs, as well as 20 MD-IPMNs formed comparison groups. Patient characteristics and symptoms at presentation were compared between min-mix-IPMN and ex-mix-IPMN or BD-IPMN and MD-IPMN (Table I). There were no observed differences in patient age and sex distribution between the min-mix-IPMN, ex-mix-IPMN, BD-IPMN, and MD-IPMN groups. In min-mix-IPMNs, the lesions were more likely to be identified incidentally compared with ex-mix-IPMNs (70% vs 29%; P < .0001) and BD-IPMNs (70% vs 50%; P = .016). Conversely, patients with ex-mix-IPMN, similar to MD-IPMNs, more frequently presented with related symptoms including weight loss (30% vs 11%; P = .009) and jaundice (14% vs 0%; P = .007) than those with min-mix-IPMN.
Table I.
Summary of patient characteristics and symptoms leading to diagnosis of intraductal mucinous neoplasm of the pancreas (IPMN) stratified by differential involvement of pancreatic ducts
| Characteristics and symptoms | Branch-duct IPMN (n = 175)
|
Mixed-type IPMN with minimal main-duct involvement (n = 46)
|
Mixed-type IPMN with extensive main-duct involvement (n = 163)
|
Main-duct IPMN (n = 20)
|
P value* | P value† | P value‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Age at diagnosis (y), median (range) | 66 | 41–92 | 65 | 39–85 | 67 | 30–67 | 70 | 40–85 | .64 | .75 | .60 |
| Sex | |||||||||||
| Male | 73 | 42 | 19 | 41 | 91 | 56 | 12 | 60 | .96 | .081 | .72 |
| Female | 102 | 58 | 27 | 59 | 72 | 44 | 8 | 40 | |||
| Smoking history | 82 | 47 | 23 | 50 | 105 | 64 | 11 | 55 | .70 | .076 | .41 |
| Diabetes mellitus | 29 | 17 | 8 | 17 | 38 | 23 | 9 | 45 | .89 | .39 | .036 |
| History of other neoplasm | 47 | 27 | 5 | 11 | 40 | 24 | 1 | 5 | .023 | .046 | .048 |
| Family history of pancreatic cancer | 5 | 11 | 27 | 15 | 26 | 16 | 2 | 10 | .43 | .39 | .48 |
| Symptoms | |||||||||||
| Abdominal pain | 87 | 50 | 14 | 30 | 81 | 50 | 11 | 55 | .020 | .021 | .65 |
| Pancreatitis | 32 | 18 | 9 | 17 | 35 | 21 | 5 | 25 | .83 | .78 | .72 |
| Weight loss (≥10 lb) | 26 | 15 | 5 | 11 | 49 | 30 | 7 | 35 | .48 | .009 | .65 |
| Jaundice | 9 | 5.1 | 0 | 0 | 23 | 14 | 4 | 20 | .116 | .007 | .48 |
| Steatorrhea | 1 | 2.2 | 10 | 5.7 | 12 | 7.4 | 1 | 5 | .33 | .20 | .70 |
| Incidental diagnosis | 87 | 50 | 32 | 70 | 48 | 29 | 0 | 0 | .016 | <.0001 | .005 |
| With other malignancy | 12 | 6.9 | 0 | 0 | 8 | 4.9 | 0 | 0 | .068 | .12 | .31 |
| Without other malignancy | 75 | 43 | 32 | 70 | 40 | 24 | 0 | 0 | <.0001 | <.0001 | <.0001 |
P value between mixed-type IPMN with minimal main-duct involvement and branch-duct IPMN.
P value between mixed-type IPMN with minimal main-duct involvement and that with extensive main-duct involvement.
P value between mixed-type IPMN with extensive main-duct involvement and main-duct IPMN.
Preoperative radiomorphologic findings
The pancreatic head was the predominant location for all IPMNs followed by the pancreatic body (Table II), but min-mix-IPMNs were more likely to involve the tail compared with ex-mix-IPMNs (22% vs 8%; P = .008). There was a trend for BD-IPMNs to present with multifocal disease (30%) compared with min-mix-IPMN (15%; P = .048), but there was no difference in multifocality between min-mix-IPMNs and ex-mix-IPMNs (15% vs 13%; P = .68). All the MD-IPMN exhibited continuous involvement of the MPD. Importantly, there were differences in the diameter of MPD between the 4 groups; the median diameter of MPD was 2 mm (range, 2–12) in min-mix-IPMN versus 9 mm (range, 2–140) in ex-mix-IPMN (P < .0001) and 2 mm (range, 2–9) in BD-IPMN (P < .0001). MD-IPMN had a median diameter of 30 mm (range, 10–90; versus ex-mix-IPMN, P = .002). One third of 45 min-mix-IPMNs demonstrated segmental prominence/dilation of MPD by imaging studies, but the involvement of MPD by mucinous epithelium was limited on microscopic examination in those cases. Similarly, there was a difference in the size of branch-duct component (cyst); the maximum diameter of the branch duct was ≥10 mm in only 62% of ex-mix-IPMNs versus 93% of min-mix-IPMNs (P < .0001) and 91% of BD-IPMNs.
Table II.
Imaging characteristics of intraductal mucinous neoplasm of the pancreas (IPMN) stratified by differential involvement of pancreatic ducts
| Characteristics | Branch-duct IPMN (n = 175)
|
Mixed-type IPMN with minimal main-duct involvement (n = 46)
|
Mixed-type IPMN with extensive main-duct involvement (n = 163)
|
Main-duct IPMN (n = 20)
|
P value* | P value† | P value‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Dominant location | |||||||||||
| Head | 113 | 65 | 34 | 74 | 98 | 60 | 11 | 55 | .008 | .22 | .20 |
| Distal | 26 | 15 | 11 | 24 | 57 | 35 | 6 | 30 | — | — | — |
| Entire pancreas | 36 | 21 | 1 | 2.2 | 8 | 4.9 | 3 | 15 | — | — | — |
| Multifocal lesions | 52 | 30 | 7 | 15 | 21 | 13 | 1 | 5 | .048 | .68 | .31 |
| Size (at operation) diameter, median, range (mm) | 25 | 6–86 | 28 | 5–60 | 22 | 5–160 | 30 | 10–90 | .73 | .77 | .56 |
| MPD component | |||||||||||
| Diameter, median, range (mm) | 2 | 2–9 | 2 | 2–12 | 9 | 2–140 | 30 | 10–90 | <.0001 | <.0001 | .002 |
| MPD dilation (≥5 mm) | |||||||||||
| Segmental | 48 | 27 | 15 | 33 | 61 | 37 | 6 | 30 | .11 | <.0001 | .13 |
| Diffuse | 0 | 0 | 1 | 2.2 | 82 | 50 | 14 | 70 | — | — | — |
| No dilation | 127 | 73 | 30 | 65 | 20 | 12 | 0 | 0 | — | — | — |
| Branch-duct component | |||||||||||
| Macroscopic (≥ 10 mm) | 160 | 91 | 43 | 93 | 101 | 62 | — | — | .651 | <.0001 | — |
| Macroscopic (<10 mm) | 15 | 8.6 | 3 | 6.5 | 42 | 26 | — | — | — | — | — |
| Microscopic only | 0 | 0 | 0 | 0 | 20 | 12 | — | — | — | — | — |
P value between mixed-type IPMN with minimal main-duct involvement and branch-duct IPMN.
P value between mixed-type IPMN with minimal main-duct involvement and that with extensive main-duct involvement.
P value between mixed-type IPMN with extensive main-duct involvement and main-duct IPMN.
MPD, Main pancreatic duct.
Indications for resection and operative procedures
Patients with min-mix-IPMN were more likely to have an observation period before operation compared with ex-mix-IPMN (41% vs 10%; P < .0001) and an increase in cyst size was the leading indication for resection in this group. Conversely, the majority of patients with ex-mix-IPMN underwent operation because of a suspicious nodule or mass (18% vs 4.3%; P = .019) and/or main-duct dilation (67% vs 17%; P < .0001). There was no difference in operative indications between min-mix-IPMN and BD-IPMN, whereas all patients with MD-IPMN underwent operation for dilation of the MPD (Table III).
Table III.
Main indications for resection of intraductal mucinous neoplasm of the pancreas (IPMN) stratified by differential involvement of pancreatic ducts
| Indications for resection | Branch-duct IPMN (n = 175)
|
Mixed-type IPMN with minimal main-duct involvement (n = 46)
|
Mixed-type IPMN with extensive main-duct involvement (n = 163)
|
Main-duct IPMN (n = 20)
|
P value* | P value† | P value‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Surgery after primary observation | 51 | 71 | 19 | 41 | 17 | 10 | 0 | 0 | .11 | <.0001 | .13 |
| MPD dilatation by imaging study | 19 | 11 | 8 | 17 | 109 | 67 | 20 | 100 | .23 | <.0001 | .002 |
| Symptoms | 36 | 21 | 7 | 15 | 29 | 18 | 8 | 40 | .41 | .68 | .02 |
| Family history of pancreatic cancer | 6 | 3.4 | 3 | 6.5 | 7 | 4.3 | 0 | 0 | .34 | .53 | .34 |
| Size or increase in size | 56 | 32 | 16 | 35 | 18 | 11 | 0 | 0 | .72 | <.0001 | .12 |
| Suspicious cytology | 35 | 20 | 9 | 20 | 17 | 10 | 0 | 0 | .95 | .10 | .13 |
| Recurrent pancreatitis | 14 | 8.0 | 6 | 13 | 29 | 18 | 5 | 25 | .29 | .45 | .43 |
| Nodule or mass | 26 | 15 | 2 | 4.3 | 30 | 18 | 3 | 15 | .057 | .019 | .71 |
P value between mixed-type IPMN with minimal main-duct involvement and branch-duct IPMN.
P value between mixed-type IPMN with minimal main-duct involvement and that with extensive main-duct involvement.
P value between mixed-type IPMN with extensive main-duct involvement and main-duct IPMN.
MPD, Main pancreatic duct.
Among all patients, a pylorus-preserving or classic pancreatoduodenectomy was performed in 245 patients (60%); 104 (26%) underwent left pancreatic resection. A total pancreatectomy was indicated and performed in 26 patients (6%), and 32 patients (8%) underwent middle pancreatectomy. In 3 cases, vascular resection and reconstruction was warranted. The median duration of stay was 7 days (range, 3–30) and the postoperative readmission rate 12%. Operative mortality was 1%, and overall morbidity was 37%. Cardiopulmonary complications were seen in 11% of patients. There were no observed differences in postoperative mortality and morbidity between the 4 groups.
Histopathologic characteristics
The results of histopathologic examination are shown in Table IV. Most importantly, the prevalence of high-grade dysplasia (37% vs 11%) and invasive carcinoma (33% vs 6.5%) was greater in ex-mix-IPMN compared with min-mix-IPMN (P < .0001), whereas the grades of dysplasia in min-mix-IPMN and BD-IPMN were comparable. The rate of invasive carcinoma was the greatest in MD-IPMN (55%). Min-mix-IPMN and ex-mix-IPMN were also different in their epithelial morphology (P < .0001). The vast majority of min-mix-IPMNs (89%) and BD-IPMNs (81%) only consisted of gastric-type epithelium, whereas almost half of ex-mix-IPMNs (47%) and 80% of MD-IPMN exhibited intestinal-type epithelium, followed by the gastric (40% and 5%, respectively) and oncocytic (4.9% and 5%, respectively) types. Similarly, all invasive carcinomas arising in min-mix-IPMN and the vast majority of those arising in BD-IPMN were of the tubular type, whereas 26% of those arising in ex-mix-IPMN and 45% of those arising in MD-IPMN were of the colloid type. The difference, however, was not significant, possibly owing to the small sample size. Invasive carcinomas that developed separately from the IPMN (concomitant pancreatic ductal adenocarcinoma) were seen in 5.7% (10/175) of BD-IPMNs, 4.3% (7/163) of ex-mix-IPMNs, and 5% (1/20) of MD-IPMNs; they were less common in min-mix-IPMNs (1/46, 2.2%).
Table IV.
Histologic characteristics of intraductal mucinous neoplasm of the pancreas (IPMN) stratified by differential involvement of pancreatic ducts
| Histologic characteristics | Branch-duct IPMN (n = 175)
|
Mixed-type IPMN with minimal main-duct involvement (n = 46)
|
Mixed-type IPMN with extensive main-duct involvement (n = 163)
|
Main-duct IPMN (n = 20)
|
P value* | P value† | P value‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Grade of dysplasia | |||||||||||
| Low | 57 | 33 | 12 | 26 | 13 | 8 | 1 | 5 | .62 | <.0001 | .15 |
| Intermediate | 80 | 46 | 26 | 56 | 35 | 21 | 5 | 25 | — | — | — |
| High | 26 | 15 | 5 | 11 | 61 | 37 | 3 | 15 | — | — | — |
| Invasive carcinoma | 12 | 7 | 3 | 6 | 54 | 33 | 11 | 55 | — | — | — |
| Epithelial subtype | |||||||||||
| Gastric | 142 | 81 | 41 | 89 | 66 | 40 | 1 | 5 | .021 | <.0001 | .030 |
| Intestinal | 28 | 16 | 3 | 6.5 | 77 | 47 | 16 | 80 | — | — | — |
| Oncocytic | 4 | 2.3 | 0 | 0 | 8 | 4.9 | 1 | 5 | — | — | — |
| Pancreatobiliary | 0 | 0 | 2 | 4.3 | 9 | 5.5 | 1 | 5 | — | — | — |
| Mixed | 1 | 0.6 | 0 | 0 | 3 | 1.8 | 1 | 5 | — | — | — |
| Invasive IPMN | |||||||||||
| With macroscopic invasion | 11 | 92 | 1 | 33 | 36 | 67 | 7 | 64 | .024 | .24 | .85 |
| With minimal invasion | 1 | 8 | 2 | 67 | 18 | 33 | 4 | 36 | — | — | — |
| T stage | |||||||||||
| pT1 | 4 | 33 | 2 | 67 | 20 | 37 | 2 | 18 | .43 | .51 | .42 |
| pT2 | 4 | 33 | 0 | 0 | 12 | 22 | 4 | 36 | — | — | — |
| pT3 | 4 | 33 | 1 | 33 | 22 | 41 | 5 | 45 | — | — | — |
| pT4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — |
| Nodal involvement | 4 | 33 | 0 | 0 | 17 | 31 | 17 | 31 | .24 | .25 | .78 |
| AJCC stage | |||||||||||
| IA | 4 | 33 | 2 | 67 | 17 | 31 | 2 | 18 | .31 | .41 | .58 |
| IB | 3 | 25 | 0 | 0 | 10 | 18 | 2 | 18 | — | — | — |
| IIA | 1 | 8.3 | 1 | 33 | 10 | 18 | 4 | 36 | — | — | — |
| IIB | 4 | 33 | 0 | 0 | 17 | 31 | 3 | 27 | — | — | — |
| III | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — |
| IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | — | — | — |
| Venous invasion (+) | 3 | 25 | 1 | 33 | 8 | 15 | 1 | 9.1 | .77 | .39 | .62 |
| Lymphatic invasion (+) | 3 | 25 | 1 | 33 | 13 | 24 | 1 | 9.1 | .77 | .72 | .27 |
| Perineural invasion (+) | 6 | 50 | 1 | 33 | 24 | 44 | 1 | 9.1 | .65 | .71 | .028 |
| Resection margin (+) | 2 | 1.1 | 0 | 0 | 33 | 20 | 5 | 25 | .47 | <.0001 | .62 |
| Histologic subtype | |||||||||||
| Tubular | 10 | 83 | 3 | 100 | 34 | 63 | 5 | 45 | .75 | .42 | .43 |
| Colloid | 1 | 8.3 | 0 | 0 | 14 | 26 | 5 | 45 | — | — | — |
| Oncocytic | 1 | 8.3 | 0 | 0 | 6 | 11 | 1 | 9.1 | — | — | — |
| Concomitant PDAC§ | 10 | 5.7 | 1 | 2.2 | 7 | 4.3 | 1 | 5 | .33 | .51 | .88 |
P value between mixed-type IPMN with minimal main-duct involvement and branch-duct IPMN.
P value between mixed-type IPMN with minimal main-duct involvement and that with extensive main-duct involvement.
P value between mixed-type IPMN with extensive main-duct involvement and main-duct IPMN.
Concomitant PDAC: Pancreatic ductal adenocarcinoma identified separately from the IPMN lesion.
AJCC, American Joint Committee on Cancer; MPD, main pancreatic duct.
When evaluating the epithelial histology of MPD versus branch ducts in min-mix-IPMN, MPD was exclusively involved by gastric-type epithelium. The main-duct components harbored lesser or the same grades of dysplasia as the cystic lesions/branch-duct components and no high-grade dysplastic epithelium was seen in MPD. In addition, MPD was noncircumferentially involved by mucinous epithelial only in 1 or 2 histologic sections in the majority (69%), and the remaining min-mix-IPMNs showed focal MPD involvement in 3 or 4 sections.
Follow-up and survival
Of all patients, 87% (n = 351) underwent regular postoperative follow-up imaging studies with a median imaging follow-up of 49 months. New IPMN lesions were observed in 7% of patients with min-mix-IPMN, not different from those in BD-IPMN (9%) and in ex-mix-IPMN (10%) or MD-IPMN (10%; P = .85). The median time to IPMN recurrence was 13 months.
After a median follow-up of 63 months, 27% of patients had died. In accordance with the greater incidence of invasive carcinoma in patients with ex-mix-IPMN, 10% of them died owing to recurrent pancreatic cancer. In patients with invasive min-mix-IPMN, only 1 died of disease progression (2.2%). Of the remaining 2 cases with invasive min-mix-IPMN, one was alive without recurrence, and the other died shortly after undergoing lung transplantation. Among patients with invasive IPMN (n = 82), 33% died because of IPMN cancer, whereas 23% died of other causes. Local carcinoma recurrence or progressive metastatic disease was observed in 33% (1/3) of patients with invasive min-mix-IPMN, and the figure was not different from that in ex-mix-IPMN (41%; P = .80) and in BD-IPMN (58%; P = .44). In MD-IPMN, cancer recurred in 36% (4/11) of malignant lesions (ex-mix-IPMN versus MD-IPMN; P = .79).
The overall 5-year survival rate after resection was 71% in min-mix-IPMN, 71% in ex-mix-IPMN, 84% in BD-IPMN, and 65% in MD-IPMN patients, and the 10-year survival rates were 71%, 56%, 66%, and 59%, respectively (min-mix-IPMN versus ex-mix-IPMN, P = .30; min-mix-IPMN versus BD-IPMN, P = .62; ex-mix-IPMN versus MD-IPMN, P = .65; Fig 2). Looking at disease-specific outcomes, the 5- and 10-year survival rates after resection were 97% and 97%, respectively, in min-mix-IPMN patients, which is better than those of ex-mix-IPMN patients (83% and 83%, respectively; P = .046), but similar to those of BD-IPMN patients (95% and 94%, respectively; P = .67). The disease-specific survival rates of MD-IPMN (69% at 5 years and 69% at 10 years) did not differ from those of ex-mix-IPMN (P = .40; Fig 3).
Fig 2.
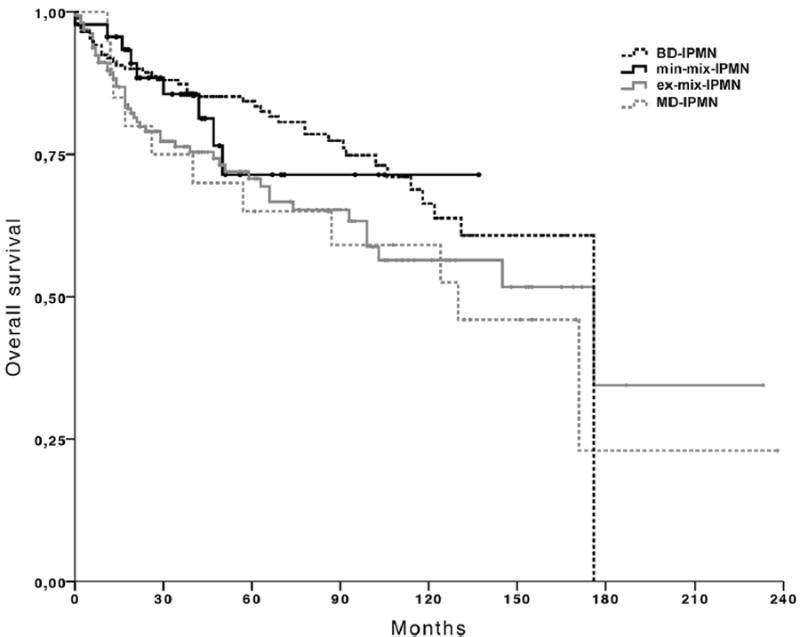
Overall survival of patients with minimal involvement of the main pancreatic duct (min-mix-IPMN), extensive involvement of the main pancreatic duct (ex-mix-IPMN), branch-duct type IPMN (BD-IPMN), and main-duct type IPMN (MD-IPMN). The overall 5-year survival rate after resection was 71% in min-mix-IPMN compared with 71% in ex-mix-IPMN, 84% in BD-IPMN and 65% in MD-IPMN patients, and the 10-year survival rates were 71%, 56%, 66% and 59%, respectively (min-mix-IPMN versus ex-mix-IPMN, P = .26; min-mix-IPMN versus BD-IPMN, P = .62; ex-mix-IPMN versus MD-IPMN, P = .65).
Fig 3.
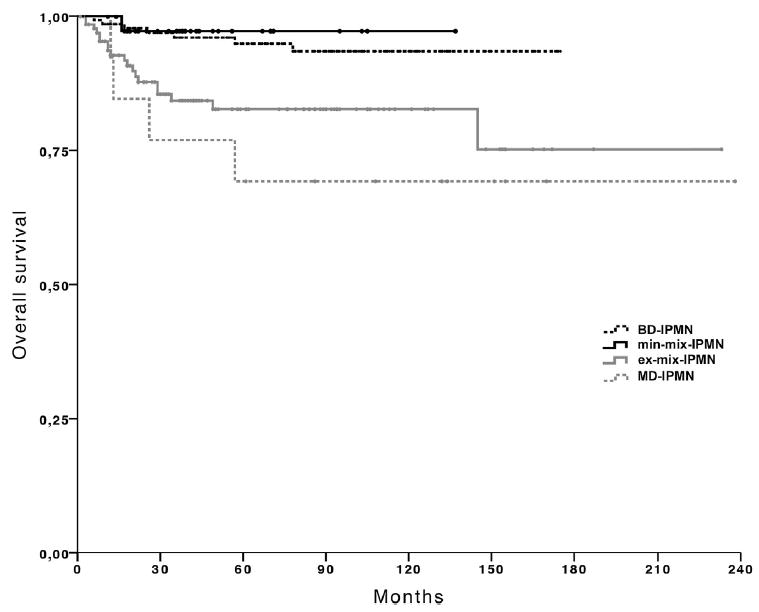
Disease-specific survival of patients minimal involvement of the main pancreatic duct (min-mix-IPMN), extensive involvement of the main pancreatic duct (ex-mix-IPMN), branch-duct type IPMN (BD-IPMN), and main-duct type IPMN (MD-IPMN). The 5- and 10-year survival rates after resection were 97% and 97%, respectively, in min-mix-IPMN patients, better than those of ex-mix-IPMN patients (83% and 83%, respectively; P = .046), but similar to those of BD-IPMN patients (95% and 94%, respectively; P = .67). Survival rates of MD-IPMN did not differ from those of ex-mix-IPMN (5-year, 69% vs 83%; 10-year, 69% vs 83%; P = .40).
DISCUSSION
In the last decade, mixed-type IPMN has been grouped together with MD-IPMN and compared with BD-IPMN.2,20,21 The picture now evolving is that mixed-type IPMN is a heterogeneous group, and much remains to be determined about the difference in biology between mixed-type IPMNs with minimal versus extensive MPD involvement. In this study, we show that min-mix-IPMN shares many clinicopathologic features and biology with BD-IPMN, and that it is different from ex-mix-IPMN.
There is no question that MPD involvement by IPMN is associated with aggressive biology. Large series describe rates of invasive cancer of 43% (range, 11–81%) in MD-IPMN compared with only 18% (range, 1.4–37%) in BD-IPMN.4,9-11 Without stratification by the extent of MPD involvement, mixed-type IPMN, as a whole, reportedly harbor the similar rate of high-grade dysplasia and invasive cancer (41%; range, 19–68%) to that of MD-IPMN.2,5,6,8-10 In our cohort, invasive carcinoma was observed only in 6% of min-mix-IPMNs compared with 33% of ex-mix-IPMN (P = .001). These differences were also reflected in disease-specific survival; patients with min-mix-IPMN followed favorable outcomes compared with those with ex-mix-IPMN (the disease-specific 5-year survival rate 97% vs 83%, respectively; P = .046).
One of the most important findings of this study is that min-mix-IPMN shared histologic characteristics with BD-IPMN. The vast majority (89%) of min-mix-IPMNs were of the gastric type, similar to BD-IPMN. Furthermore, the main-duct components of min-mix-IPMNs consisted exclusively of the gastric-type epithelium, even in those with the branch-duct component exhibiting the intestinal (16%) or pancreatobiliary (4%) epithelium. Importantly, the main-duct components harbored lesser or the same grades of dysplasia as the cystic lesions/branch-duct components and no high-grade dysplasia was identified in MPD. Conversely, in ex-mix-IPMNs, intestinal, pancreatobiliary and/or oncocytic type epithelium often involves MPD leading to MPD dilation and/or mural nodules (data not shown). Given that the gastric-type epithelium is associated with a low risk of malignant transformation and ultimately indolent biology,15,17 it is no surprise to see favorable outcomes of patients with min-mix-IPMN compared with ex-mix-IPMN.11,15,22
Patients with IPMN can be diagnosed incidentally during the workup for other conditions or present with variable symptoms. In our cohort, all patients with MD-IPMN presented with IPMN-related symptoms, whereas the half of BD-IPMNs were incidental findings, the figures similar to those of the previous reports.5,12,20,23 In our min-mix-IPMN cohort, 70% were found incidentally, and 41% underwent operation after primary observation. Increased size of the cyst was the leading indication for the operation similar to BD-IPMNs. Conversely, 61% of ex-mix-IPMNs presented with related symptoms, and the incidence of weight loss and jaundice in ex-mix-IPMNs was similar to that of MD-IPMNs, but was significantly greater than min-mix-IPMNs and BD-IPMNs.
MPD involvement by IPMN may lead to the dilation of MPD owing to hypersecretion of mucin and/or protein plugs or can only be identified on microscopic examination without any macroscopic abnormalities. In one study of pancreatic cysts found incidentally, preoperative imaging study failed to identify MPD abnormalities in up to 40% of mixed-type IPMNs.24 We observed that ex-mix-IPMN showed MPD dilatation in 87%, whereas in min-mix-IPMN imaging studies described MPD dilatation in only 33%. In addition, most min-mix-IPMN appeared with a predominant branch-duct component of ≥10 mm in diameter. Thus, 65% of min-mix-IPMNs were diagnosed as BD-IPMN at the time of presentation and, as discussed, a substantial number of cases were put on surveillance initially without immediate operative intervention. The results of our study assure that the difficulty of distinguishing min-mix-IPMN from BD-IPMN preoperatively has no clinical relevance, because their biology seems to be comparable. Conversely, given that the vast majority of min-mix-IPMNs exhibited low- or intermediate-grade dysplasia of the gastric type, especially those resected only owing to MPD dilation by imaging studies and/or an increase in size (38%) could have been followed without operative intervention. In those cases, endoscopic ultrasonography-guided cyst fluid aspiration for cytologic interpretation and/or biomarker analysis may play a role in confirming the benign nature of the lesion.25,26
It is yet to be determined whether min-mix-IPMNs represent a distinct entity or a progression from BD-IPMN to ex-mix-IPMN. We did not observe a difference in the median age of diagnosis between min-mix-IPMN (65 years) versus ex-mix-IPMN (67 years; P = .75) in our cohort; however, in some min-mix-IPMNs, MPD with focal involvement by mucinous epithelium was associated immediately with a large branch duct that was involved circumferentially by mucinous epithelium with a greater grade of dysplasia (data not shown), raising the possibility of IPMN extending from peripheral ducts to MPD. The fact that a large proportion of min-mix-IPMNs were resected owing to an increase in size of the cysts after primary observation also supports the possibility. Similarly, ex-mix-IPMN share many clinicopathologic features, including an high prevalence of intestinal-type epithelium, and prognosis with MD-IPMN, suggesting that at least some ex-mix-IPMNs are predominantly MD-IPMNs with branch-duct involvement.
Shortcomings of our study include the retrospective nature with a nonstandardized sampling of the main duct in histologic examination and the limited sample size of min-mix-IPMN, even in this large series of resected IPMNs. More standardized sampling with several sections of normal-appearing MPD may have increased the number of min-mix-IPMNs by reclassifying some cases from BD-IPMN to min-mix-IPMN. The results of this study, however, would not have changed, because min-mix-IPMNs and BD-IPMNs shared similar clinicopathologic characteristics and prognosis.
In conclusion, our experience with a large single-center cohort of resected IPMNs reveals that about one fourth (22%) of all mixed-type IPMNs only exhibit minimal main-duct involvement. Two thirds of these cases have no dilation or any other MPD abnormality by imaging studies, and the MPD involvement is an incidental finding by microscopic examination. Min-mix-IPMN shares histopathologic characteristics and less aggressive biology with BD-IPMN. At present, it is uncertain whether min-mix-IPMN represents a state of progression from BD-IPMN to ex-mix-IPMN or whether it is a distinct entity within the heterogeneous group of IPMN. The results of our study suggest that min-mix-IPMN should be classified differently from MD- and ex-mix-IPMNs.
Acknowledgments
S.P.T. was supported by National Cancer Institute grants P01 CA117969 and R01 CA169086. M.M.-K. was supported by National Cancer Institute grants P50 CA127003 and R01 CA169086.
References
- 1.Itai Y, Ohhashi K, Nagai H, Murakami Y, Kokubo T, Makita K, et al. “Ductectatic” mucinous cystadenoma and cystade-nocarcinoma of the pancreas. Radiology. 1986;161:697–700. doi: 10.1148/radiology.161.3.3786719. [DOI] [PubMed] [Google Scholar]
- 2.Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol. 2000;17:16–30. [PubMed] [Google Scholar]
- 4.Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in traductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–9. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241–6. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708–13. 713.e1-2. doi: 10.1053/j.gastro.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–51. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–9. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bournet B, Kirzin S, Carrere N, Portier G, Otal P, Selves J, et al. Clinical fate of branch duct and mixed forms of intra-ductal papillary mucinous neoplasia of the pancreas. J Gastroenterol Hepatol. 2009;24:1211–7. doi: 10.1111/j.1440-1746.2009.05826.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanno A, Satoh K, Hirota M, Hamada S, Umino J, Itoh H, et al. Prediction of invasive carcinoma in branch type intra-ductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010;45:952–9. doi: 10.1007/s00535-010-0238-0. [DOI] [PubMed] [Google Scholar]
- 12.Crippa S, Fernandez-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Dominguez I, et al. Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol. 2010;8:213–9. doi: 10.1016/j.cgh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Chari S, Adsay V, Fernandez-Del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 15.Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, Valsangkar NP, Liss AS, Hsu M, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on his-tological and precursor epithelial subtypes. Gut. 2011;60:1712–20. doi: 10.1136/gut.2010.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa T, Kloppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, et al. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509–16. doi: 10.1136/gut.2010.210567. [DOI] [PubMed] [Google Scholar]
- 18.Hruban RH, P M, Klimstra DS. Tumors of the pancreas. Annapolis Junction (MD): American Registry of Pathology; 2007. [Google Scholar]
- 19.Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, Coban I, et al. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 2010;34:364–70. doi: 10.1097/PAS.0b013e3181cf8bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvia R, Crippa S, Partelli S, Armatura G, Malleo G, Paini M, et al. Differences between main-duct and branch-duct in-traductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:342–6. doi: 10.4240/wjgs.v2.i10.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohri D, Asaoka Y, Ijichi H, Miyabayashi K, Kudo Y, Seto M, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol. 2012;47:203–13. doi: 10.1007/s00535-011-0482-y. [DOI] [PubMed] [Google Scholar]
- 22.Nakata K, Ohuchida K, Aishima S, Sadakari Y, Kayashima T, Miyasaka Y, et al. Invasive carcinoma derived from intestinal-type intraductal papillary mucinous neoplasm is associated with minimal invasion, colloid carcinoma, and less invasive behavior, leading to a better prognosis. Pancreas. 2011;40:581–7. doi: 10.1097/MPA.0b013e318214fa86. [DOI] [PubMed] [Google Scholar]
- 23.Woo SM, Ryu JK, Lee SH, Yoon WJ, Kim YT, Yoon YB. Branch duct intraductal papillary mucinous neoplasms in a retrospective series of 190 patients. Br J Surg. 2009;96:405–11. doi: 10.1002/bjs.6557. [DOI] [PubMed] [Google Scholar]
- 24.Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernandez-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10:144–50. doi: 10.1159/000243733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genevay M, Mino-Kenudson M, Yaeger K, Konstantinidis IT, Ferrone CR, Thayer S, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg. 2011;254:977–83. doi: 10.1097/SLA.0b013e3182383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das KK, Xiao H, Geng X, Fernandez-Del-Castillo C, Morales-Oyarvide V, Daglilar E, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN) Gut. 2013 Nov 25; doi: 10.1136/gutjnl-2013-306219. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


