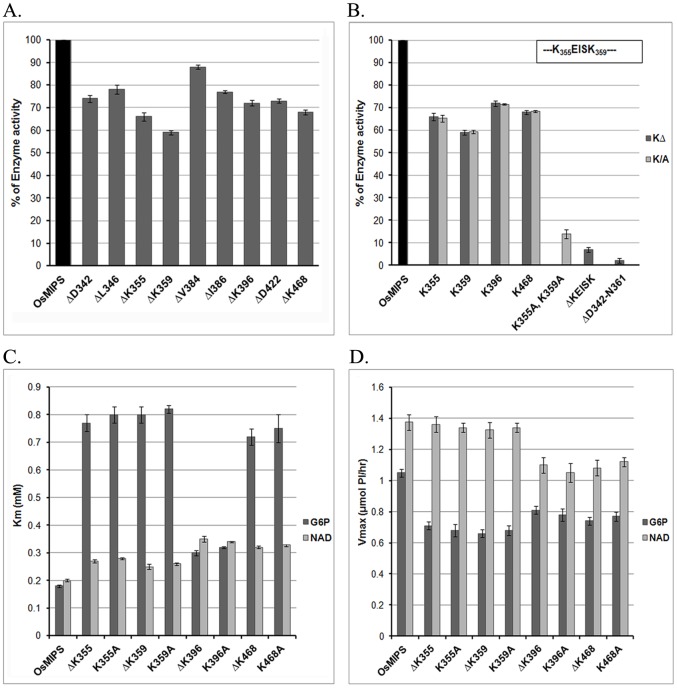
Fig 5. Comparison of enzyme activity of wild OsMIPS protein and its mutants.
(A) Residual MIPS activity of deletion mutants with respect to the wild type enzyme OsMIPS. Black arrows indicate the four lysine deletion mutants having least enzyme activity. (B) Residual MIPS activity of deletion and replacement mutants of lysine residues within the catalytic domain represented as KΔ and K/A, respectively. ΔKEISK represents the pentapeptide (inset) deletion mutant while K355A, K359A represents double substitution at positions 355 and 359 of OsMIPS. (C) Km and (D) Vmax values for the substrate, G6P and cofactor, NAD, as calculated from Lineweaver-Burk plot.