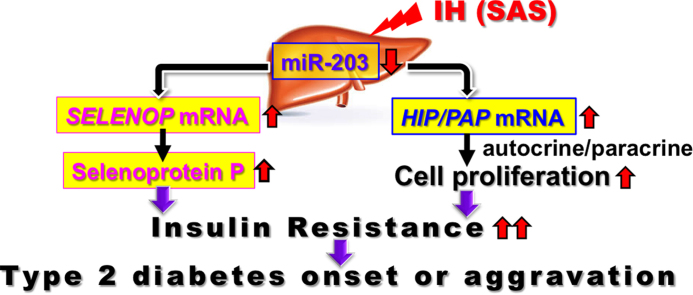
Abstract
Sleep apnea syndrome is characterized by recurrent episodes of oxygen desaturation and reoxygenation (intermittent hypoxia [IH]) and is a risk factor for insulin resistance/type 2 diabetes. However, the mechanisms linking IH stress and insulin resistance remain elusive. We exposed human hepatocytes (JHH5, JHH7, and HepG2) to experimental IH or normoxia for 24 h, measured mRNA levels by real-time reverse transcription polymerase chain reaction (RT-PCR), and found that IH significantly increased the mRNA levels of selenoprotein P (SELENOP) — a hepatokine — and hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein (HIP/PAP) — one of REG (Regenerating gene) family. We next investigated promoter activities of both genes and discovered that they were not increased by IH. On the other hand, a target mRNA search of micro RNA (miRNA) revealed that both mRNAs have a potential target sequence for miR-203. The miR-203 level of IH-treated cells was significantly lower than that of normoxia-treated cells. Thus, we introduced miR-203 inhibitor and a non-specific control RNA (miR-203 inhibitor NC) into HepG2 cells and measured the mRNA levels of SELENOP and HIP/PAP. The IH-induced expression of SELENOP and HIP/PAP was abolished by the introduction of miR-203 inhibitor but not by miR-203 inhibitor NC. These results demonstrate that IH stress up-regulates the levels of SELENOP in human hepatocytes to accelerate insulin resistance and up-regulates the levels of HIP/PAP mRNAs to proliferate such hepatocytes, via the miR-203 mediated mechanism.
Abbreviations: AHSG, α2 HS-glycoprotein; ANGPTL6, angiopoietin-related growth factor; DROSHA, ribonuclease type III; DICER, endoribonuclease Dicer; ELISA, enzyme-linked immunosorbent assay; FCS, fetal calf serum; FGF21, fibroblast growth factor 21; HIP/PAP, hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein; IH, intermittent hypoxia; LECT2, leukocyte cell-derived chemotaxin 2; MCPIP1, monocyte chemotactic protein-induced protein 1; miRNA, micro RNA; Reg, regenerating gene; Rig, rat insulinoma gene; RpS15, ribosomal protein S15; SAS, sleep apnea syndrome; SELENOP, selenoprotein P; SHBG, sex hormone-binding globulin; siRNA, small interfering RNA; TP63, transformation-related protein 63; WST-8, 2-(2-methoxy-4-nitrophenyl)−3-(4-nitrophenyl)−5-(2,4-disulfophenyl)−2H-tetrazolium monosodium salt
Keywords: Hepatokine, HIP/PAP, Intermittent hypoxia, miR-203, REG family gene, SELENOP
Graphical abstract
1. Introduction
Sleep apnea syndrome (SAS) is characterized by the narrowing or collapse of the upper airway during sleep that leads to a cessation of airflow. Apnea and hypopnea are often accompanied by a drop in oxygen saturation. Accumulating evidence suggests that recurrent episodes of oxygen desaturation and reoxygenation (intermittent hypoxia [IH]), which are typical features of SAS, contribute to the development of β cell dysfunction and impaired glucose tolerance [1].
Epidemiological and clinical evidence postulates that SAS may be a causal factor of type 2 diabetes. The increasing severity of SAS is associated with worsening insulin resistance [2], [3]. The recent report by Priou et al. suggested that an increase in the severity of SAS may worsen glucose control in patients with asymptomatic, untreated, or early stages of type 2 diabetes [4]. Nocturnal IH is associated with an increased risk of type 2 diabetes among community-dwelling Japanese people independent of its traditional risk factors such as age, sex, and body habitus [5]. However, the mechanisms by which IH induces insulin resistance in SAS patients are not well established.
Recently, several proteins that are exclusively or predominantly secreted from the liver, called hepatokines, were established as directly affecting glucose and lipid metabolism [6], [7]. For example, fibroblast growth factor 21 (FGF21) has recently emerged as a novel hormone, leading to beneficial effects on glucose metabolism and lipid homeostasis [8], while selenoprotein P is correlated positively with insulin resistance and could be a therapeutic target for type 2 diabetes [9]. The changes of these hepatokine expressions in hepatocytes by IH remain elusive.
The regenerating gene (Reg) was identified in regenerating islets [10], [11] and a Reg gene product — Reg protein — acts as a growth factor and promotes cell proliferation and regeneration [11], [12], [13]. In humans, five functional Reg family genes (REG Iα, REG Iβ, REG III, hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein [HIP/PAP] and REG IV) have been isolated. For several cells, Reg family proteins have been suggested to be involved in cellular proliferation [11]. We have reported that IH stress stimulates pancreatic β cell proliferation via up-regulation of Reg family mRNAs and may cause hyperinsulinemia, which makes patients more obese [1], [14]. However, the direct effects of IH on hepatocyte proliferation and the IH-induced changes in Reg family gene expression in hepatocytes remain unknown.
In the present study, we investigated the changes of gene expression in hepatokines and Reg family genes, as well as their regulation mechanisms in response to IH stress in hepatocytes.
2. Materials and methods
2.1. Cell culture
Rat H4IIE hepatocytes were grown in DMEM medium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) containing 10% (v/v) fetal calf serum (FCS), 100 units/mL penicillin G (Wako) and 100 μg/mL streptomycin (Wako) as described [15]. Human hepatocarcinoma JHH5 cells and JHH7 cells were purchased from Japanese Collection of Research Bioresources (Sennan, Japan) and human hepatocarcinoma HepG2 cells were purchased from RIKEN BRC CELL BANK (Tsukuba, Japan). JHH5 and JHH7 cells were grown in William's E medium (Sigma, St. Louis, MO) containing 10% (v/v) FCS, 100 units/mL penicillin G (Wako), and 100 μg/mL streptomycin (Wako), and HepG2 cells were grown in DMEM medium containing 10% (v/v) FCS, 100 units/mL penicillin G, and 100 μg/mL streptomycin as described [16]. Cells were exposed to either normoxia (21% O2, 5% CO2, and balance N2) or intermittent hypoxia (IH: 64 cycles of 5 min sustained hypoxia [1% O2, 5% CO2, and balanced N2] and 10 min normoxia) using a custom-designed, computer-controlled incubation chamber attached to an external O2-CO2-N2 computer-driven controller (O2 programmable control, 9200EX, Wakenyaku CO., Ltd, Kyoto, Japan), as described [1], [16]. These conditions are similar to the conditions reported in patients with severe degree of SAS — in severe cases of SAS, patients are repeatedly exposed to severe hypoxemia followed by mild hypoxemia or normoxia (i.e., IH). We previously reported that the magnitude of IH expressed by SpO2 fluctuated between 75–98% and 50–80% in SAS [1], [17], which was almost equivalent to the medium condition in the present study.
2.2. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated using a RNA protect cell mini kit (Qiagen, Hilden, Germany) from JHH5, JHH7, HepG2, and H4IIE cells, and cDNA was synthesized from total RNA as template using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) as described [14], [16], [18], [19], [20]. Real-time polymerase chain reaction (PCR) was performed using SYBR® Fast qPCR kit (KAPA Biosystems, Boston, MA) and a Thermal Cycler Dice Real Time System (Takara, Kusatsu, Japan). All the PCR primers were synthesized by Nihon Gene Research Laboratories, Inc. (NGRL; Sendai, Japan), and the primer sequences for each primer set are described in Table 1. PCR was performed with an initial step of 3 min at 95 °C followed by 40 cycles of 3 s at 95 °C and 20 s at 60 °C for β-actin, rat insulinoma gene (Rig)/ribosomal protein S15 (RpS15), REG III, and HIP/PAP, 40 cycles of 3 s at 95 °C and 20 s at 64 °C for REG Iα, REG Iβ, and REG IV, 45 cycles of 3 s at 95 °C and 20 s at 60 °C for α2 HS-glycoprotein (AHSG), angiopoietin-related growth factor 6 (ANGPTL6), FGF21, leukocyte cell-derived chemotaxin 2 (LECT2), LIPASIN, sex hormone-binding globulin (SHBG), Selenoprotein P (SELENOP), ribonuclease type III (DROSHA), endoribonuclease Dicer (DICER), monocyte chemotactic protein-induced protein 1 (MCPIP1), transformation-related protein p63 (TP63), and miR-203. The mRNA expression levels were normalized to the mRNA level of Rig/RpS15 in rat samples or β-actin in human samples, and the miR-203 level was normalized to the U6 RNA level.
Table 1.
Primers used for real-time RT-PCR.
| Target mRNA/miR | Primer sequence |
|---|---|
| Rat Selenop (NM_001082911) | 5′-GACAGTGGTTGCTCTTCTTCAA-3′ |
| 5′-TCGCAGGTCTTCCAATCTG-3′ | |
| Rat Rig/RpS15 (NM_017151) | 5′-ACGGCAAGACCTTCAACCAG-3′ |
| 5′-ATGGAGAACTCGCCCAGGTAG-3′ | |
| Human SELENOP (NM_001093726) | 5′-TCATCAAGGAATCTCTTCTCG-3′ |
| 5′-CAAGACGGCCACATCTATCA-3′ | |
| Human ANGPTL6 (NM_031917) | 5′-CTGTGGTTCCGGTCCGTCTT-3′ |
| 5′-GCTGCTCACACCATACTGACACT-3′ | |
| Human SHBG (NM_001040) | 5′-TCAATCTCCGAGACATTCCC-3′ |
| 5′-TGGTGTCCCAAGAGCAAG-3′ | |
| Human LIPASIN (NM_018687) | 5′-GGCCGCACAATAGAACTCC-3′ |
| 5′-CAGCGTGAGCCTTTAAGACC-3′ | |
| Human LECT2 (NM_002302) | 5′-GTGTTCGAATATCTGGAAGAGGT-3′ |
| 5′-AAGGGCAATAGAGTTCCAAGT-3′ | |
| Human FGF21 (NM_019113) | 5′-ACCTGGAGATCAGGGAGGAT-3′ |
| 5′-AGTGGAGCGATCCATACAGG-3′ | |
| Human AHSG (NM_001622) | 5′-CCCCGGAAAACACGCACA-3′ |
| 5′-GTGCCAAACCTCCTCATCTCT-3′ | |
| Human REG Iα (NM_002909) | 5′-AGGAGAGTGGCACTGATGACTT-3′ |
| 5′-TAGGAGACCAGGGACCCACTG-3′ | |
| Human REG Iβ (NM_006507) | 5′-GCTGATCTCCTCCCTGATGTTC-3′ |
| 5′-GGCAGCTGATTCGGGGATTA-3′ | |
| Human REG III (AB161037) | 5′-GAATATTCTCCCCAAACTG-3′ |
| 5’-GAGAAAAGCCTGAAATGAAG-3′ | |
| Human HIP/PAP (NM_138937) | 5′-AGAGAATATTCGCTTAATTCC-3′ |
| 5′-AATGAAGAGACTGAAATGACA-3′ | |
| Human REG IV (AY007243) | 5′-ATCCTGGTCTGGCAAGTC-3′ |
| 5′-CGTTGCTGCTCCAAGTTA-3′ | |
| Human SOCS3 (NM_003955) | 5′-CCACTCTTCAGCATCTCTGT-3′ |
| 5′-ATCGTACTGGTCCAGGAACT-3′ | |
| Human TP63 (NM_003722) | 5′-TGTATCCGCATGCAGGACT-3′ |
| 5′-CTGTGTTATAGGGACTGGTGGAC-3′ | |
| Human DICER (NM_177438) | 5′-GAGCTGTCCTATCAGATCAGGG-3′ |
| 5′-ACTTGTTGAGCAACCTGGTTT-3′ | |
| Human DROSHA (NM_013235) | 5′-GGCCCGAGAGCCTTTTATAG-3′ |
| 5′-TGCACACGTCTAACTCTTCCAC-3′ | |
| Human MCPIP1 (NM_025079) | 5′-TGCCTATCACAGACCAGCAC-3′ |
| 5′-CTCACCTTCGCGAAGTAGCTC-3′ | |
| Human β-actin (NM_001101) | 5′-GCGAGAAGATGACCCAGA-3′ |
| 5′-CAGAGGCGTACAGGGATA-3′ | |
| Human miR-203 (NR_029620) | 5′-GCCGGTGAAATGTTTAGGAC-3′ |
| 5′-GTGCAGGGTCCGAGGT-3′ | |
| Human U6 (NR_004394) | 5′-CTCGCTTCGGCAGCACA-3′ |
| 5′-AACGCTTCACGAATTTGCGT-3′ |
2.3. Measurement of Sepp1 in culture medium by enzyme-linked immunosorbent assay (ELISA)
Cells were exposed either normoxia or IH for 24 h, culture medium was collected, and the concentration of Sepp1 was measured by using a Human Selenoprotein P (SELENOP) ELISA kit (Cusabio, Wuhan, China) for human cells and Rat Selenoprotein P (Selenop) ELISA kit (Cusabio) according to the instructions of supplier.
2.4. Measurement of viable cell numbers by tetrazolium salt cleavage
HepG2 cells (2.5 × 104 cells/100 µL in 96-well plate) were incubated at 37 °C over night and the medium was replaced with DMEM+10% FCS just before normoxia/IH exposure. After a 24-h treatment of normoxia or IH, the viable cell numbers were determined by a Cell Counting kit-8 (Dojindo Laboratories, Mashiki-machi, Japan) according to the manufacturer's instructions. Briefly, WST-8 (2-(2-methoxy-4-nitrophenyl)−3-(4-nitrophenyl)−5-(2,4-disulfophenyl)−2H-tetrazolium monosodium salt) solution was added to cells in 96-well plates, and the cells were incubated at 37 °C for 30 min. The optical density of each well was read at 450 nm (reference wave length at 650 nm) using a SunriseTM microplate reader (Tecan, Männedorf, Switzerland), as described [14], [20].
2.5. RNA interference
Small interfering RNA (siRNA) directed against human HIP/PAP and REG Iα were synthesized by NGRL. The sense sequences of siRNA for human HIP/PAP and REG Iα were 5’-GUGAAGAGCAUUGGUAACAGCUAtt-3’ (corresponding 335–357 of NM_002580) and 5’-GCAAUUACAACGGAGUCAAtt-3’ (corresponding 640–658 of NM_002909), respectively. The Silencer® Select human scrambled siRNA was purchased from Ambion® and used as a control. Transfection of siRNAs into HepG2 cells was carried out using Lipofectamine® RNAiMAX Transfection Reagent (Life Technologies). Cells were transfected with 1 pmol each of siRNA in a 96-well culture dish as described [14], [18], [19], [20], and viable cell numbers were analyzed by WST-8 assay as described above.
2.6. Construction of reporter plasmid and luciferase assay
Reporter plasmids were prepared by inserting the promoter fragments of human SELENOP (−2989~+10) and HIP/PAP (−4030~+27) upstream of a firefly luciferase reporter gene in the pGL4.17 vector (Promega, Madison, WI). The reporter plasmids were transfected into human HepG2 hepatocytes using Lipofectamine® 3000 (Invitrogen), as described [19], [20], and the cells were exposed to either 64 cycles/24 h of IH, mimicking hepatocytes of SAS patients, or normoxia for 24 h. After cells were exposed to IH, cells were lysed and promoter activities were measured. The cells were harvested and cell extracts were prepared in Extraction Buffer (0.1 M potassium phosphate, pH 7.8/0.2% Triton X-100; Life Technologies). To monitor transfection efficiency, pCMV-SPORT-βgal plasmid (Life Technologies) was co-transfected in all experiments at a 1:10 dilution. Luciferase activity was measured using a PicaGene luciferase assay system (Toyo-ink, Tokyo, Japan) and was normalized by the β-galactosidase activity as described previously [15], [16], [18], [19], [20], [21], [22], [23].
2.7. MiRNA extraction, reverse transcription, and real-time quantitative PCR
Total RNA including miRNA was isolated from HepG2 cells using the miRNeasy mini kit (Qiagen) according to the manufacturer's instructions. An equal amount of DNase-treated RNA was Poly-A tailed using a Mir-XTM miRNA first strand synthesis kit (Clonetech) according to the manufacturer's protocol. The condition for PCR was 95 °C for 10 s, followed by 45 cycles of amplification (95 °C, 5 s, 60 °C, 20 s). U6 small nuclear RNA was used as an endogenous control for miRNA. The primers are listed in Table 1.
2.8. MiR-203 inhibitor transfection
MiR-203 inhibitor (5′-CUAGUGGUCCUAAACAUUUCAC-3′) and non-specific control RNA (miR-203 inhibitor NC) (5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by NGRL and introduced into HepG2 cells using Lipofectamine® RNAiMAX (Invitrogen) [18], [19], [20] just before IH/normoxia exposure, and the mRNA levels of SELENOP and HIP/PAP were measured by real-time RT-PCR, as described [14], [16], [18], [19], [20].
2.9. Data analysis
Results are expressed as mean ± SE. Statistical significance was determined by Student's t-test using GraphPad Prism software (GraphPad Software, La Jolla, CA).
3. Results
3.1. SEPP1 gene expression was increased by IH
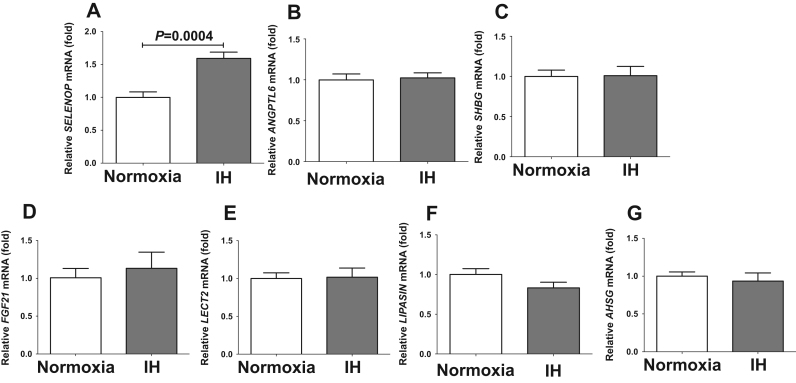
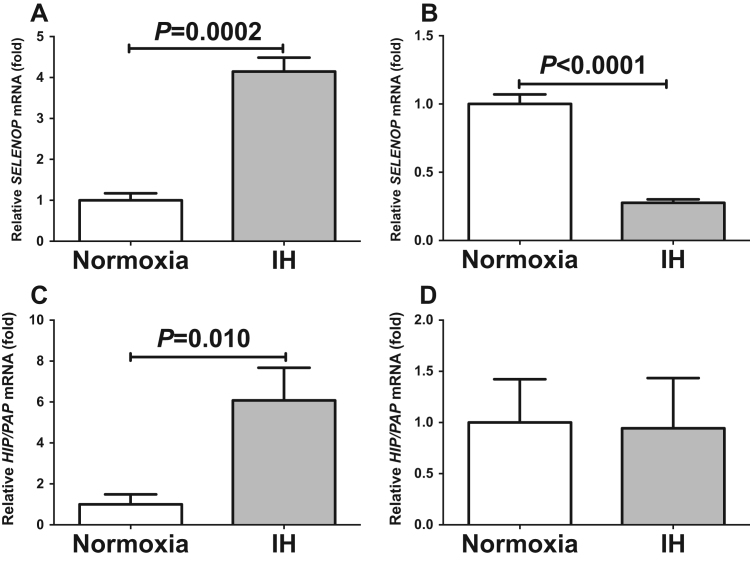
Hepatokines, secretory proteins mainly produced by the liver, were reported to correlate with glucose homeostasis and insulin resistance [6], [7]. We analyzed the mRNA levels of several hepatokines affecting glucose metabolism (SELENOP, ANGPTL6, SHBG, FGF21, LIPASIN, LECT2, and AHSG) by real-time RT-PCR in IH and normoxia-treated hepatocytes. The mRNA levels of Selenop were significantly increased by IH in all the analyzed hepatocytes: HepG2 (P=0.0004; Fig. 1), JHH5 (P=0.0477; Supplementary Fig. 1), JHH7 (P=0.0027; Supplemental Fig. 2), and H4IIE (P=0.0057; Supplementary Fig. 3). However, there was no hepatokine other than Selenop that increased in all the four tested cells in response to IH stimulation (Fig. 1 and Supplementary Figs. 1–3). These results indicate that IH stress specifically up-regulates expression of SELENOP in hepatocytes.
Fig. 1.
The mRNA levels of SELENOP (A), ANGPTL6 (B), SHBG (C), FGF21 (D), LECT2 (E), LIPASIN (F), and AHSG (G) in HepG2 cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using β-actin as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student's t-test.
We further measured Selenop protein in the culture medium by ELISA and found that the levels of Selenop were significantly increased by IH in all the analyzed hepatocytes: HepG2 (P=0.0256; Fig. 2), JHH5 (P=0.0370; Supplementary Fig. 4 A), JHH7 (P=0.0434; Supplementary Fig. 4B), and H4IIE (P=0.048; Supplementary Fig. 4 C).
Fig. 2.
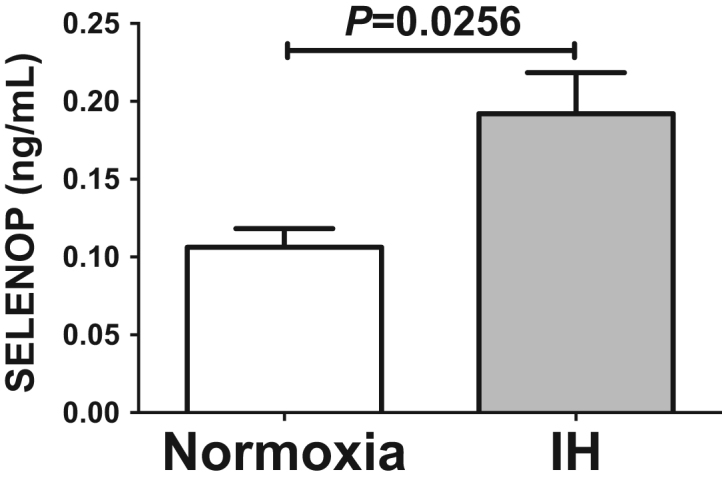
Concentrations of SELENOP in HepG2 cell culture medium were measured by ELISA. HepG2 cells were treated by normoxia or IH conditions for 24 h. Data is expressed as means ± SE for each group (n=4).
3.2. HIP/PAP gene expression was increased by IH
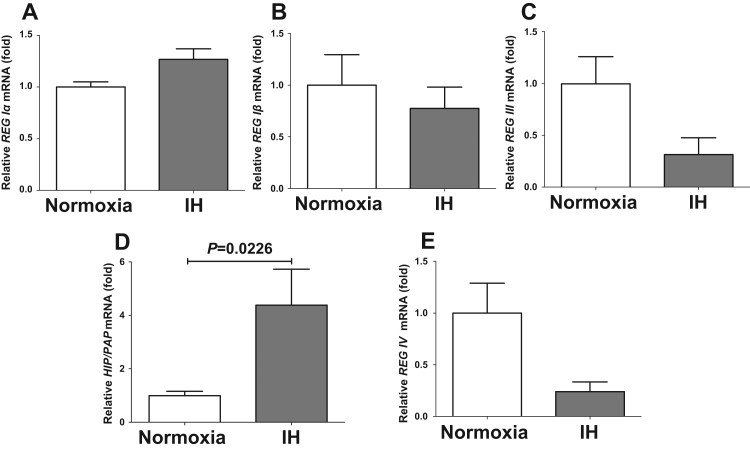
The Reg gene was originally identified in regenerating pancreatic islets, and the Reg gene and its related genes (Reg family genes) were revealed to encode growth factors for several cells [10], [11], [12]. We have reported that IH stress induced β cell proliferation via up-regulation of Reg family genes [1], [14]. In this study, we analyzed the changes of REG family gene expression in human hepatocytes by IH. The mRNA levels of HIP/PAP were significantly increased in IH-treated human hepatocytes: HepG2 (P=0.0226: Fig. 3), JHH5 (P=0.0022: Supplementary Fig. 5), and JHH7 (P=0.0016: Supplementary Fig. 6). Meanwhile, mRNA levels of the other REG family members (REG Iα, REG Iβ, REG III, and REG IV) were not increased (Fig. 3). Except for REG IV in JHH5 (Supplementary Fig. 5), the mRNA levels of other REG family genes were not increased by IH (Supplementary Figs. 5 and 6).
Fig. 3.
The mRNA levels of REG Iα (A), REG Iβ (B), REG III (C), HIP/PAP (D), and REG IV (E) in HepG2 cells treated by normoxia or IH for 24 h were measured by real-time RT-PCR. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student's t-test.
3.3. HIP/PAP acts as an autocrine/paracrine growth factor for hepatocytes in IH condition
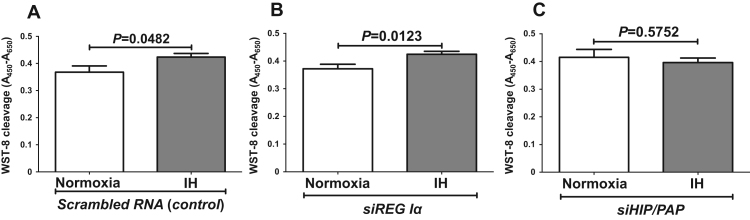
In order to evaluate direct effects of IH on hepatocyte proliferation, HepG2 cells were exposed to normoxia or IH for 24 h. After the treatment, cell viability was determined by WST-8 assay. HepG2 cell proliferation was significantly increased by IH (Fig. 4). Furthermore, RNA interference of HIP/PAP inhibited HepG2 cell proliferation measured by WST-8 assay, whereas interference of REG Iα did not (Fig. 5). These results indicate that HIP/PAP, up-regulated by IH, works as an autocrine/paracrine growth factor in hepatocyte proliferation.
Fig. 4.
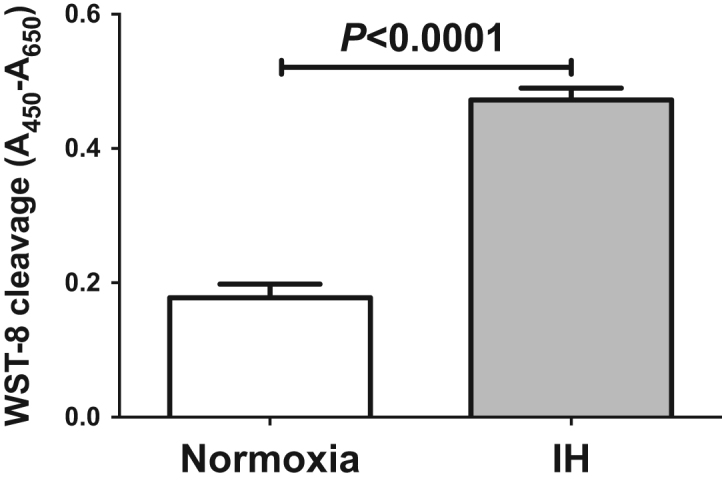
Effects of IH on cellular proliferation of HepG2 cells. They were exposed to normoxia or IH conditions for 24 h, and cellular proliferation was measured by WST-8 assay. Data is expressed as means ± SE for each group (n=12). The statistical analyses were performed using Student's t-test.
Fig. 5.
Effects of siRNA transfection on cell proliferation. After transfection of siRNA against REG Iα or HIP/PAP into HepG2 cells, cells were exposed to normoxia or IH conditions for 24 h, and cellular proliferation was measured by WST-8 assay. Data is expressed as means ± SE for each group (n=12). The statistical analyses were performed using Student's t-test.
3.4. The promoter activities of SELENOP and HIP/PAP were not increased by IH
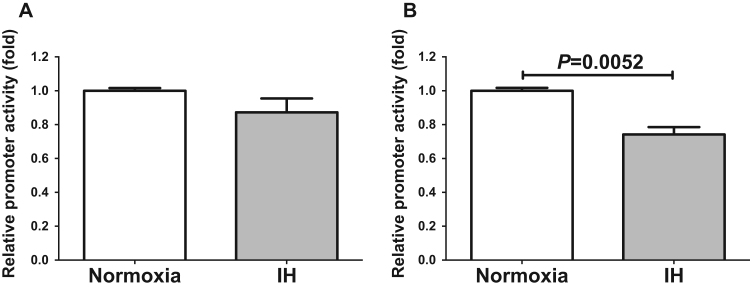
To determine whether the IH-induced increases in SELENOP and HIP/PAP mRNA were caused by activation of transcription, a 2999 bp fragment containing 2989 bp of the SELENOP promoter and a 4057 bp fragment containing 4030 bp of HIP/PAP promoter were fused to the luciferase gene of pGL4.17 and transfected into HepG2 cells. After IH stimulation, we measured promoter activities and found that both SELENOP and HIP/PAP promoter activities actually decreased due to IH in HepG2 cells (Fig. 6). In addition, promoter activities of SELENOP and HIP/PAP did not increased due to IH in JHH5 and JHH7 cells (Supplementary Fig. 7). These results strongly suggest that the gene expression of both SELENOP and HIP/PAP in response to IH was not regulated by transcription.
Fig. 6.
Luciferase assays in HepG2 cells. Reporter plasmids prepared by inserting the promoter fragments of SELENOP (−2989~+10) and HIP/PAP (−4030~+27) upstream of a firefly luciferase reporter gene in the pGL4.17 vector were transfected into HepG2 cells. After cells were exposed to 24 h of either IH or normoxia, the cells were lysed, and promoter activities of SELENOP (A) and HIP/PAP (B) were measured. All data is represented as the mean ± SE of the samples (n=3). The statistical analyses were performed using Student's t-test.
3.5. The miR-203 level was significantly decreased by IH
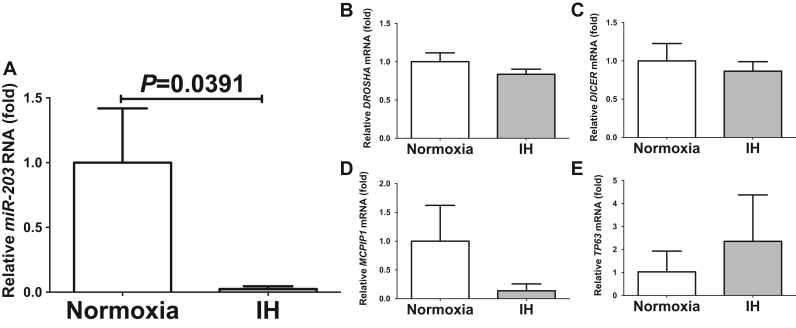
As the IH-induced up-regulation of SELENOP and HIP/PAP was considered to be regulated post-transcriptionally, we searched targeted miRNA using the MicroRNA.org program (http://www.microrna.org/microrna/home.do), which revealed that both SELENOP and HIP/PAP mRNAs have a potential target sequence for miR-203. There were no other miRNA candidates targeting both genes. There are some controversial reports concerning occurrence of miR-203 in mammalian liver cells: Sonkoly et al. reported miR-203 was abundant in skin but scarcely detected in liver [24]. Szele et al. reported miR-203 occurrence in liver was 0.614–0.616 using 5 S RNA as an internal control [25]. We measured the miR-203 level of IH-treated cells by RT-PCR and found that the level was significantly lower than that of normoxia-treated cells (0.0270±0.02058 fold vs normoxia, P=0.0391). There are two possibilities as to why the level of miR-203 was decreased by IH. One possibility is that IH influences mRNA levels of some enzymes for miRNA biosynthesis/degradation. Another is that the level of miR-203 was specifically decreased by IH either via decreased biosynthesis or enhanced degradation in response to IH. We measured the mRNA levels of DROSHA, DICER, MCPIP1, and TP63, which are involved in the biosynthesis and degradation of miRNAs [26], [27], [28] and found that their expression was unchanged by IH (Fig. 7). These results suggest that miR-203 plays a key role in post-transcriptional regulation of mRNA levels of SELENOP and HIP/PAP. To investigate whether SELENOP and HIP/PAP expression in IH is regulated by miR-203, miR-203 inhibitor and non-specific control RNA (miR-203 inhibitor NC) were introduced into HepG2 cells just before IH/normoxia exposure, and the mRNA levels of SELENOP and HIP/PAP were measured by real-time RT-PCR. As shown in Fig. 8, the IH-induced increases in SELENOP and HIP/PAP mRNAs were abolished by the introduction of miR-203 inhibitor but not by miR-203 inhibitor NC. We also introduced miR-203 mimic into HepG2 cells, exposed the cells with IH, measured mRNA levels, and found that the IH-induced increases in SELENOP and HIP/PAP mRNAs were abolished by the introduction of miR-203 mimic but not by non-specific control RNA (miR-203 mimic NC) (Supplementary Fig. 8). These findings indicate that IH stress down-regulates the miR-203 level in human hepatocytes and that the levels of SELENOP and HIP/PAP mRNAs are increased via the miR-203 mediated mechanism.
Fig. 7.
The mRNA levels of miR-203 (A), DROSHA (B), DICER (C), MCPIP1 (D), and TP63 (E) in HepG2 cells treated by normoxia or IH for 24 h. The levels of mRNAs were measured by real-time RT-PCR using β-actin as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student's t-test.
Fig. 8.
Effects of miR-203 inhibitor transfection on SELENOP and HIP/PAP expression. The miR-203 inhibitor and non-specific control RNA (miR-203 inhibitor NC) were introduced into HepG2 cells just before normoxia or IH exposure. The expression of SELENOP and HIP/PAP mRNA was measured by real-time RT-PCR using β-actin as an endogenous control. The figure represents (A) SELENOP mRNA expression in miR-203 inhibitor NC-introduced cells, (B) SELENOP mRNA expression in miR-203 inhibitor-introduced cells, (C) HIP/PAP mRNA expression in miR-203 inhibitor NC-introduced cells and (D) HIP/PAP mRNA expression in miR-203 inhibitor-introduced cells. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student's t-test.
4. Discussion
Recent epidemiological research demonstrates that SAS may be associated with hypertension, dyslipidemia, cardiovascular diseases and insulin resistance, suggesting that SAS patients may be susceptible to various metabolic dysfunctions. In particular, SAS is closely associated with type 2 diabetes, independent of obesity and family history [2], [29]. Kendzerska et al. reported that the initial SAS severity predicted risk for diabetes [3] and Punjabi and Beamer reported that mild, moderate, and severe SAS patients displayed a 26.7%, 36.5%, and 43.7% reduction in insulin sensitivity, respectively, after adjusting for age, sex, race, and body fat percentage [29]. Moreover, the intervention with continuous positive airway pressure for SAS treatment could improve not only SAS itself but also diabetes [2], [30], [31].
The repeated hypoxia and reoxygenation cycles, IH, which are induced by upper airway collapse, are a characteristic feature of SAS patients. Recent studies revealed that the nocturnal IH is correlated with an increased risk of developing type 2 diabetes [5]. Although the mechanisms linking IH stress to insulin resistance remain to be elusive, several explanations have been proposed, such as activating the sympathetic nervous system, inducing the release of cathecholamines, producing reactive oxygen species, and activating inflammatory pathways [2], [31], [32], [33]. Hyperglycemia in type 2 diabetes patients is the result of impaired insulin secretion and increased insulin resistance in target tissues — liver, skeletal muscle, adipose tissue, etc. Recently, like adipose tissue and skeletal muscle, the liver is known to affect glucose metabolism by releasing proteins into circulation, which are termed hepatokines [6]. In the present study, to investigate the direct effect of IH on the expression of hepatokines in hepatocytes, we measured several mRNAs for hepatokine (ANGPTL6, SHBG, FGF21, LIPASIN, LECT2, AHSG, and SELENOP) that were reported to play critical roles in glucose homeostasis [8], [34], [35], [36], [37], [38]. We found that the SELENOP mRNA level was significantly increased in all the hepatocytes, whereas the other hepatokines were not (Fig. 1, Supplementary Figs. 1–3). Furthermore, the concentrations of selenoprotein P in all the hepatocyte culture media were significantly increased by IH. Selenoprotein P was first characterized to contain selenium as selenocysteine, and it plays a central role in selenium homeostasis [39]. Recently, Misu et al. revealed that the serum levels of selenoprotein P and gene expression of SELENOP in human liver were increased in type 2 diabetes patients [6] and that Selenop knockout mice showed increases responsiveness to insulin/exercise [9]. Our results indicated that IH stress up-regulates the mRNA levels of selenoprotein P in hepatocytes, suggesting the possible contribution of selenoprotein P in SAS patients to aggravate insulin resistance.
HIP/PAP is originally found as a gene overexpressed in liver carcinoma [40] and the pancreas during the acute phase of pancreatitis [41]. Recent HIP/PAP transgenic/knockout mouse experiments [42], [43], [44] revealed that it is an autocrine/paracrine mitogenic factor, accelerating liver regeneration. HIP/PAP promotes cell proliferation in hepatocytes by protecting them from apoptosis and acting as a mitogenic factor [42], [44]. Based on the primary structure of encoded protein, HIP/PAP is considered to be a member of the REG family gene [11]. We evaluated cellular proliferation by WST-8 assay and found that HepG2 cell proliferation was significantly increased by IH (P<0.0001) compared to that by normoxia (Fig. 4). Furthermore, we measured the mRNA levels of all the REG family genes (REG Iα, REG Iβ, REG III, HIP/PAP and REG IV) and found that HIP/PAP was significantly increased by IH (Fig. 3, Supplementary Fig. 5). This result suggests that IH stress up-regulates the mRNA levels of HIP/PAP in human hepatocytes and may stimulate the proliferation of liver cells with high levels of SELENOP mRNA. In fact, the knockdown experiment using siRNA against HIP/PAP mRNA showed that HIP/PAP acts as an autocrine/paracrine growth factor for hepatocytes stimulated by IH (Fig. 5). Recently, putative links between SAS and increased cancer incidence were reported [45], [46], suggesting possible involvement of up-regulation of HIP/PAP as a growth factor for cancer cells. In addition, HIP/PAP has recently been recognized as an obesogenic factor [47] in addition to a growth factor. It may be possible that HIP/PAP in addition to SELENOP is involved in insulin resistance via obesity.
Next, we investigated the mechanisms by which IH up-regulates the mRNA levels of SELENOP and HIP/PAP and found that the promoter activities of SELENOP and HIP/PAP were not increased by IH (Fig. 6, Supplementary Fig. 7). This suggests that IH-induced up-regulation of SELENOP and HIP/PAP mRNAs (Fig. 1, Fig. 3, Supplementary Figs. 1–3, 5 and 6) was regulated by post-transcriptional step. MiRNAs are short non-coding RNAs that modulate gene expression via post-transcriptional regulation in many cellular processes [48]. They promote RNA degradation and inhibit transcription by binding to mRNA and affecting its stability, resulting in the regulation of the amount of mRNAs. Some miRNAs have specific individual targets, while others regulate hundreds of mRNA levels simultaneously [49], [50]. The role of miRNA in the regulation of numerous biological processes (angiogenesis, apoptosis, cell proliferation, migration, etc.) for various types of cancer has been well-established. Additionally, accumulating evidence indicates the importance of the roles of miRNAs in metabolism, such as regulating cholesterol, lipid metabolism, and controlling insulin signaling [51], [52], [53], [54]. In the current study, miR-203 with common target sequence in SELENOP and HIP/PAP mRNAs could contribute to worsening glucose intolerance in IH-conditions by up-regulation SELENOP and HIP/PAP mRNA.
In conclusion, the present study revealed that the gene expression of SELENOP and HIP/PAP were increased via down-regulation of miR-203 level in IH-treated hepatocytes. In SAS patients, it is suggested that the up-regulation of SELENOP plays a role in worsening insulin resistance. In addition, overexpression of HIP/PAP could proliferate such hepatocytes in SAS patients, leading to decreased insulin sensitivity and much more.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan and is in partial fulfillment by T. Uchiyama of the degree of Doctor of Medical Science at Nara Medical University.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2017.07.005.
Appendix A. Supplementary material
Supplementary material Supplementary Fig. 1. The mRNA levels of SELENOP (A), ANGPTL6 (B), SHBG (C), FGF21 (D), LECT2 (E), LIPASIN (F) and AHSG (G) in JHH5 cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using β-actin as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 2. The mRNA levels of SELENOP (A), ANGPTL6 (B), SHBG (C), FGF21 (D), LECT2 (E), LIPASIN (F) and AHSG (G) in JHH7 cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using β-actin as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 3. The mRNA levels of Selenop (A), Angptl6 (B), Shbg (C), Fgf21 (D), Lect2 (E), Lipasin (F) and Ahsg (G) in H4IIE cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using Rig/RpS15 as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 4. Concentrations of Selenop in the culture medium were measured by ELISA. (A) JHH5, (B) JHH7, and (C) H4IIE cells were treated with normoxia or IH for 24 h. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 5. The mRNA levels of REG Iα (A), REG Iβ (B), REG III (C), HIP/PAP (D) and REG IV (E) in JHH5 cells treated by normoxia or IH for 24 h were measured by real-time RT-PCR. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 6. The mRNA levels of REG Iα (A), REG Iβ (B), REG III (C), HIP/PAP (D) and REG IV (E) in JHH7 cells treated by normoxia or IH for 24 h were measured by real-time RT-PCR. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 7. Luciferase assays in JHH5 (A and B) and JHH7 cells (C and D). Reporter plasmids prepared by inserting the promoter fragments of SELENOP (-2989~+10) and HIP/PAP (-4030~+27) upstream of a firefly luciferase reporter gene in pGL4.17 vector were transfected into JHH5 and JHH7 cells. After cells were exposed to 24 h of either IH or normoxia, the cells were lysed, and promoter activities of SELENOP (A and C) and HIP/PAP (B and D) were measured. All data is represented as the mean ± SE of the samples (n=3). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 8. Effects of miR-203 mimic transfection on SELENOP and HIP/PAP expression. The miR-203 mimic (5’-GUGAAAUGUUUAGGACCACUAG-3’) and non-specific control RNA (miR-203 mimic NC) (5’-UUCUCCGAACGUGUCACGUtt-3’) were synthesized by NGRL and introduced into HepG2 cells using Lipofectamine® RNAiMAX [18-20] just before IH/normoxia exposure, and the mRNA levels of SELENOP and HIP/PAP were measured by real-time RT-PCR, as described [14, 16, 18-20]. The expression of SELENOP and HIP/PAP mRNA was measured by real-time RT-PCR using β-actin as an endogenous control. The figure represents (A) SELENOP mRNA expression in miR-203 mimic NC-introduced cells, (B) SELENOP mRNA expression in miR-203 mimic-introduced cells, (C) HIP/PAP mRNA expression in miR-203 mimic NC-introduced cells and (D) HIP/PAP mRNA expression in miR-203 mimic-introduced cells. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test.
References
- 1.Ota H., Takasawa S., Yamauchi M., Yoshikawa M., Tomoda K., Kimura H. Intermittent hypoxia in pancreatic beta cells. Pancreat. Disord. Ther. 2015;5:S5–004. [Google Scholar]
- 2.Tasali E., Ip M.S.M. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc. Am. Thorac. Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 3.Kendzerska T., Gershon A.S., Hawker G., Tomlinson G., Leung R.S. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am. J. Respir. Crit. Care Med. 2014;190:218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 4.Priou P., Le Vaillant M., Meslier N., Chollet S., Pigeanne T., Masson P., Bizieux-Thaminy A., Humeau M.P., Goupil F., Ducluzeau P.H., Gagnadoux F. The IRSR sleep control group, association between obstructive sleep apnea severity and glucose control in patients with untreated versus treated diabetes. J. Sleep Res. 2015;24:425–431. doi: 10.1111/jsr.12278. [DOI] [PubMed] [Google Scholar]
- 5.Muraki I., Tanigawa T., Yamagishi K., Sakurai S., Ohira T., Imano H., Kitamura A., Kiyama M., Sato S., Shimamoto T., Konishi M., Iso H. CIRCS investigators, Nocturnal intermittent hypoxia and the development of type 2 diabetes: the circulatory risk in communities study (CIRCS) Diabetologia. 2010;53:481–488. doi: 10.1007/s00125-009-1616-0. [DOI] [PubMed] [Google Scholar]
- 6.Misu H., Takamura T., Takayama H., Hayashi H., Matsuzawa-Nagata N., Kurita S., Ishikura K., Ando H., Takeshita Y., Ota T., Sakurai M., Yamashita T., Mizukoshi E., Yamashita T., Honda M., Miyamoto K., Kubota T., Kubota N., Kadowaki T., Kim H.J., Lee I.K., Minokoshi Y., Saito Y., Takahashi K., Yamada Y., Takakura N., Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Yang S.J., Hwang S.Y., Choi H.Y., Yoo H.J., Seo J.A., Kim S.G., Kim N.H., Baik S.H., Choi D.S., Choi K.M. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: Implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 2011;96:E1325–E1329. doi: 10.1210/jc.2011-0620. [DOI] [PubMed] [Google Scholar]
- 8.Yu J., Yu B., Jiang H., Chen D. Conjugated linoleic acid induces hepatic expression of fibroblast growth factor 21 through PPAR-α. Br. J. Nutr. 2012;107:461–465. doi: 10.1017/S0007114511003205. [DOI] [PubMed] [Google Scholar]
- 9.Misu H., Takayama H., Saito Y., Mita Y., Kikuchi A., Ishii K., Chikamoto K., Kanamori T., Tajima N., Lan F., Takeshita Y., Honda M., Tanaka M., Kato S., Matsuyama N., Yoshioka Y., Iwayama K., Tokuyama K., Akazawa N., Maeda S., Takekoshi K., Matsugo S., Noguchi N., Kaneko S., Takamura T. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017;23:508–516. doi: 10.1038/nm.4295. [DOI] [PubMed] [Google Scholar]
- 10.Terazono K., Yamamoto H., Takasawa S., Shiga K., Yonemura Y., Tochino Y., Okamoto H. A novel gene activated in regenerating islets. J. Biol. Chem. 1988;263:2111–2114. [PubMed] [Google Scholar]
- 11.Takasawa S. Regenerating gene (REG) product and its potential clinical usage. Exp. Opin. Ther. Targets. 2016;20:541–550. doi: 10.1517/14728222.2016.1123691. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T., Yonemura Y., Yonekura H., Suzuki Y., Miyashita H., Sugiyama K., Moriizumi S., Unno M., Tanaka O., Kondo H., Bone A.J., Takasawa S., Okamoto H. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg protein. Proc. Natl. Acad. Sci. USA. 1994;91:3589–3592. doi: 10.1073/pnas.91.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shervani N.J., Takasawa S., Uchigata Y., Akiyama T., Nakagawa K., Noguchi N., Takada H., Takahashi I., Yamauchi A., Ikeda T., Iwamoto Y., Nata K., Okamoto H. Autoantibodies to REG, a beta-cell regeneration factor, in diabetic patients. Eur. J. Clin. Invest. 2004;34:752–758. doi: 10.1111/j.1365-2362.2004.01419.x. [DOI] [PubMed] [Google Scholar]
- 14.Ota H., Itaya-Hironaka A., Yamauchi A., Sakuramoto-Tsuchida S., Miyaoka T., Fujimura T., Tsujinaka H., Yoshimoto K., Nakagawara K., Tamaki S., Takasawa S., Kimura H. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013;93:664–672. doi: 10.1016/j.lfs.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Murakami-Kawaguchi S., Takasawa S., Onogawa T., Nata K., Itaya-Hironaka A., Sakuramoto-Tsuchida S., Yamauchi A., Ota H., Takeda M., Kato M., Okamoto H. Expression of Ins1 and Ins2 genes in mouse fetal liver. Cell Tissue Res. 2014;355:303–314. doi: 10.1007/s00441-013-1741-4. [DOI] [PubMed] [Google Scholar]
- 16.Ota H., Tamaki S., Itaya-Hironaka A., Yamauchi A., Sakuramoto-Tsuchida S., Morioka T., Takasawa S., Kimura H. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci. 2012;90:206–211. doi: 10.1016/j.lfs.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Niijima M., Kimura H., Edo H., Shinozaki T., Kang J., Masuyama S., Tatsumi K., Kuriyama T. Manifestation of pulmonary hypertension during REM sleep in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 1999;159:1766–1772. doi: 10.1164/ajrccm.159.6.9808064. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi A., Itaya-Hironaka A., Sakuramoto-Tsuchida S., Takeda M., Yoshimoto K., Miyaoka T., Fujimura T., Tsujinaka H., Tsuchida C., Ota H., Takasawa S. Synergistic activations of REG Iα and REG Iβ promoters by IL-6 and glucocorticoids through JAK/STAT pathway in human pancreatic β cells. J. Diabetes Res. 2015;2015:173058. doi: 10.1155/2015/173058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimura T., Fujimoto T., Itaya-Hironaka A., Miyaoka T., Yoshimoto K., Yamauchi A., Sakuramoto-Tsuchida S., Kondo S., Takeda M., Tsujinaka H., Azuma M., Tanaka Y., Takasawa S. Interleukin-6/STAT pathway is responsible for the induction of gene expression of REG Iα, a new auto-antigen in Sjögren's syndrome patients, in salivary duct epithelial cells. Biochem. Biophys. Rep. 2015;2:69–74. doi: 10.1016/j.bbrep.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujinaka H., Itaya-Hironaka A., Yamauchi A., Sakuramoto-Tsuchida S., Ota H., Takeda M., Fujimura T., Takasawa S., Ogata N. Human retinal pigment epithelial cell proliferation by the combined stimulation of hydroquinone and advanced glycation end-products via up-regulation of VEGF gene. Biochem. Biophys. Rep. 2015;2:123–131. doi: 10.1016/j.bbrep.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.T. Akiyama, S. Takasawa, K. Nata, S. Kobayashi, M. Abe, N.J. Shervani, T. Ikeda, K. Nakagawa, M. Unno, S. Matsuno, H. Okamoto, Activation of Reg gene, a gene for insulin-producing β-cell regeneration: Poly (ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)ation, Proc. Natl. Acad. Sci. U.S.A. 98 (2001) pp. 48–53. [DOI] [PMC free article] [PubMed]
- 22.Nakazawa T., Takasawa S., Noguchi N., Nata K., Tohgo A., Mori M., Nakagawara K., Akiyama T., Ikeda T., Yamauchi A., Takahashi I., Yoshikawa T., Okamoto H. Genomic organization, chromosomal localization, and promoter of human gene for FK506-binding protein 12.6. Gene. 2005;360:55–64. doi: 10.1016/j.gene.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Takasawa S., Ikeda T., Akiyama T., Nata K., Nakagawa K., Shervani N.J., Noguchi N., Murakami-Kawaguchi S., Yamauchi A., Takahashi I., Tomioka-Kumagai T., Okamoto H. Cyclin D1 activation through ATF-2 in Reg-induced pancreatic β-cell regeneration. FEBS Lett. 2006;580:585–591. doi: 10.1016/j.febslet.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 24.Sonkoly E., Wei T., Janson P.C., Sääf A., Lundeberg L., Tengvall-Linder M., Norstedt G., Alenius H., Homey B., Scheynius A., Ståhle M., Pivarcsi A. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS ONE. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szele E., Gombos K., Juhász K., Wohler V., Kovács A., Ember I. Effects of purified glycerol from biodiesel on miRNAs compared to the expression profile of selected mRNAs in Balb/c mice. In Vivo. 2013;27:107–111. [PubMed] [Google Scholar]
- 26.Zhang J., Zhang X.H., Wang C.X., Liu B., Fan X.S., Wen J.J., Shi Q.L., Zhou X.J. Dysregulation of MicroRNA biosynthesis enzyme Dicer plays an important role in gastric cancer progression. Int. J. Clin. Exp. Pathol. 2014;7:1702–1707. [PMC free article] [PubMed] [Google Scholar]
- 27.Francia S., Michelini F., Sexena A., Tang D., de Hoon M., Anelli V., Mione M., Carninci P., d’Adda di Fagagna F. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature. 2012;488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattiske S., Ho K., Noll J.E., Neilsen P.M., Callen D.F., Suetani R.J. TAp63 regulates oncogenic miR-155 to mediate migration and tumour growth. Oncotarget. 2013;4:1894–1903. doi: 10.18632/oncotarget.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punjabi N.M., Beamer B.A. Alterations in glucose disposal in sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasche K., Keller T., Tautz B., Hader C., Hergenç G., Antosiewicz J., Di Giulio C., Pokorski M. Obstructive sleep apnea and type 2 diabetes. Eur. J. Med. Res. 2010;15(Suppl. 2):152–156. doi: 10.1186/2047-783X-15-S2-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong W., Tang Y.G., Zhao X., Go F.Y., Harper R.M., Hui H. Treating obstructive sleep apnea with continuous positive airway pressure benefits type 2 diabetes management. Pancreas. 2014;43:325–330. doi: 10.1097/MPA.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi M., Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid. Redox Signal. 2008;10:755–768. doi: 10.1089/ars.2007.1946. [DOI] [PubMed] [Google Scholar]
- 33.Tamaki S., Yamauchi M., Fukuoka A., Makinodan K., Koyama N., Tomoda K., Yoshikawa M., Kimura H. Nocturnal hypoxic stress activates invasive ability of monocytes in patients with obstructive sleep apnoea syndrome. Respirology. 2009;14:689–694. doi: 10.1111/j.1440-1843.2009.01540.x. [DOI] [PubMed] [Google Scholar]
- 34.Oike Y., Akao M., Yasunaga K., Yamauchi T., Morisada T., Ito Y., Urano T., Kimura Y., Kubota Y., Maekawa H., Miyamoto T., Miyata K., Matsumoto S., Sakai J., Nakagata N., Takeya M., Koseki H., Ogawa Y., Kadowaki T., Suda T. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat. Med. 2005;11:400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 35.Chikamoto K., Misu H., Takayama H., Kikuchi A., Ishii K., Lan F., Takata N., Tajima-Shirasaki N., Takeshita Y., Tsugane H., Kaneko S., Matsugo S., Takamura T. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice. Biochem. Biophys. Res. Commun. 2016;478:1310–1316. doi: 10.1016/j.bbrc.2016.08.117. [DOI] [PubMed] [Google Scholar]
- 36.Andersen G., Burgdorf K.S., Sparsø T., Borch-Johnsen K., Jørgensen T., Hansen T., Pedersen O. AHSG tag single nucleotide polymorphisms associate with type 2 diabetes and dyslipidemia: Studies of metabolic traits in 7,683 white Danish subjects. Diabetes. 2008;57:1427–1432. doi: 10.2337/db07-0558. [DOI] [PubMed] [Google Scholar]
- 37.Fu Z., Berhane F., Fite A., Seyoum B., Abou-Samra A.B., Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci. Rep. 2014;4:5013. doi: 10.1038/srep05013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding E.L., Song Y., Manson J.E., Hunter D.J., Lee C.C., Rifai N., Buring J.E., Gaziano J.M., Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burk R.F., Hill K.E., Selenoprotein, An P. extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 2005;25:215–235. doi: 10.1146/annurev.nutr.24.012003.132120. [DOI] [PubMed] [Google Scholar]
- 40.Lasserre C., Christa L., Simon M.T., Vernier P., Bréchot C. A novel gene (HIP) activated in human primary liver cancer. Cancer Res. 1992;52:5089–5095. [PubMed] [Google Scholar]
- 41.Orelle B., Keim V., Masciotra L., Dagorn J.C., Iovanna J.L. Human pancreatitis-associated protein. Messenger RNA cloning and expression in pancreatic diseases. J. Clin. Invest. 1992;90:2284–2291. doi: 10.1172/JCI116115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieu H.T., Batteux F., Simon M.T., Cortes A., Nicco C., Zavala F., Pauloin A., Tralhao J.G., Soubrane O., Weill B., Bréchot C., Christa L. HIP/PAP accelerates liver regeneration and protects against acetaminophen injury in mice. Hepatology. 2005;42:618–626. doi: 10.1002/hep.20845. [DOI] [PubMed] [Google Scholar]
- 43.Gironella M., Folch-Puy E., LeGoffic A., Garcia S., Christa L., Smith A., Tebar L., Hunt S.P., Bayne R., Smith A.J.H., Dagorn J.-C., Closa D., Iovanna J.L. Experimental acute pancreatitis in PAP/HIP knock-out mice. Gut. 2007;56:1091–1097. doi: 10.1136/gut.2006.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon M.T., Pauloin A., Normand G., Lieu H.T., Mouly H., Pivert G., Carnot F., Tralhao J.G., Brechot C., Christa L. HIP/PAP stimulates liver regeneration after partial hepatectomy and combines mitogenic and anti-apoptotic functions through the PKA signaling pathway. FASEB J. 2003;17:1441–1450. doi: 10.1096/fj.02-1013com. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-García M.Á., Campos-Rodríguez F., Almendros I., Farré R. Relationship between sleep apnea and cancer. Arch. Bronconeumol. 2015;51:456–461. doi: 10.1016/j.arbres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Gozal D., Farré R., Nieto F.J. Putative links between sleep apnea and cancer: From hypotheses to evolving evidence. Chest. 2015;148:1140–1147. doi: 10.1378/chest.15-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Secq V., Mallmann C., Gironella M., Lopez B., Closa D., Garcia S., Christa L., Montalto G., Dusetti N., Iovanna J.L. PAP/HIP protein is an obesogenic factor. J. Cell. Physiol. 2014;229:225–231. doi: 10.1002/jcp.24438. [DOI] [PubMed] [Google Scholar]
- 48.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 49.Bao B., Wang Z., Li Y., Kong D., Ali S., Banerjee S., Ahmad A., Sarkar F.H. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim. Biophys. Acta. 1815;2011:135–146. doi: 10.1016/j.bbcan.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slezak-Prochazka I., Durmus S., Kroesen B.-J., van den Berg A. MicroRNAs, macrocontrol: Regulation of miRNA processing. RNA. 2010;16:1087–1095. doi: 10.1261/rna.1804410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou X., Liu W., Gu M., Zhou H., Zhang G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J. Gastroenterol. 2015;50:1027–1040. doi: 10.1007/s00535-015-1051-6. [DOI] [PubMed] [Google Scholar]
- 52.Rottiers V., Näär A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latouche C., Natoli A., Reddy-Luthmoodoo M., Heywood S.E., Armitage J.A., Kingwell B.A. MicroRNA-194 modulates glucose metabolism and its skeletal muscle expression is reduced in diabetes. PLoS One. 2016;11:e0155108. doi: 10.1371/journal.pone.0155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolfschoten I.G.M., Roggli E., Nesca V., Regazzi R. Role and therapeutic potential of microRNAs in diabetes. Diabetes Obes. Metab. 2009;11:118–129. doi: 10.1111/j.1463-1326.2009.01118.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Supplementary Fig. 1. The mRNA levels of SELENOP (A), ANGPTL6 (B), SHBG (C), FGF21 (D), LECT2 (E), LIPASIN (F) and AHSG (G) in JHH5 cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using β-actin as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 2. The mRNA levels of SELENOP (A), ANGPTL6 (B), SHBG (C), FGF21 (D), LECT2 (E), LIPASIN (F) and AHSG (G) in JHH7 cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using β-actin as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 3. The mRNA levels of Selenop (A), Angptl6 (B), Shbg (C), Fgf21 (D), Lect2 (E), Lipasin (F) and Ahsg (G) in H4IIE cells treated by normoxia or IH for 24 h. The levels of the hepatokine mRNAs were measured by real-time RT-PCR using Rig/RpS15 as an endogenous control. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 4. Concentrations of Selenop in the culture medium were measured by ELISA. (A) JHH5, (B) JHH7, and (C) H4IIE cells were treated with normoxia or IH for 24 h. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 5. The mRNA levels of REG Iα (A), REG Iβ (B), REG III (C), HIP/PAP (D) and REG IV (E) in JHH5 cells treated by normoxia or IH for 24 h were measured by real-time RT-PCR. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 6. The mRNA levels of REG Iα (A), REG Iβ (B), REG III (C), HIP/PAP (D) and REG IV (E) in JHH7 cells treated by normoxia or IH for 24 h were measured by real-time RT-PCR. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 7. Luciferase assays in JHH5 (A and B) and JHH7 cells (C and D). Reporter plasmids prepared by inserting the promoter fragments of SELENOP (-2989~+10) and HIP/PAP (-4030~+27) upstream of a firefly luciferase reporter gene in pGL4.17 vector were transfected into JHH5 and JHH7 cells. After cells were exposed to 24 h of either IH or normoxia, the cells were lysed, and promoter activities of SELENOP (A and C) and HIP/PAP (B and D) were measured. All data is represented as the mean ± SE of the samples (n=3). The statistical analyses were performed using Student’s t-test. Supplementary Fig. 8. Effects of miR-203 mimic transfection on SELENOP and HIP/PAP expression. The miR-203 mimic (5’-GUGAAAUGUUUAGGACCACUAG-3’) and non-specific control RNA (miR-203 mimic NC) (5’-UUCUCCGAACGUGUCACGUtt-3’) were synthesized by NGRL and introduced into HepG2 cells using Lipofectamine® RNAiMAX [18-20] just before IH/normoxia exposure, and the mRNA levels of SELENOP and HIP/PAP were measured by real-time RT-PCR, as described [14, 16, 18-20]. The expression of SELENOP and HIP/PAP mRNA was measured by real-time RT-PCR using β-actin as an endogenous control. The figure represents (A) SELENOP mRNA expression in miR-203 mimic NC-introduced cells, (B) SELENOP mRNA expression in miR-203 mimic-introduced cells, (C) HIP/PAP mRNA expression in miR-203 mimic NC-introduced cells and (D) HIP/PAP mRNA expression in miR-203 mimic-introduced cells. Data is expressed as means ± SE for each group (n=4). The statistical analyses were performed using Student’s t-test.