Abstract
Since 1989, the USA has been pursuing the goal of tuberculosis elimination. After substantial progress during the past two decades, the rate of tuberculosis cases in the USA each year has now levelled off and remains well above the elimination threshold. Both epidemiological data and modelling underline the necessity of addressing latent tuberculosis infection if further progress is to be made in eliminating the disease. In this Personal View we explore next steps towards elimination. Given the estimated prevalence of latent tuberculosis infection, compared with the limited testing and treatment that currently occur, a major new effort is required. This effort should consist of a surveillance system or registry to monitor progress, scale-up of targeted testing for latent tuberculosis infection in at-risk populations, scale-up of short-course treatment regimens, engagement of affected communities and medical providers who serve those communities, and increased public health staffing for implementation and oversight. Such an effort would benefit greatly from the development of new tools, such as tests that better indicate reactivation risk, and even shorter latent tuberculosis infection treatment regimens than currently exist.
Introduction
After the development of WHO’s End TB Strategy for the global elimination of tuberculosis, WHO and the European Respiratory Society convened a group to draft a framework for tuberculosis elimination in low-incidence countries.1,2 Although elimination of tuberculosis in these countries will not have the greatest effect on global elimination of tuberculosis, lessons learned in this context are likely to be relevant to countries with high disease burden in the future. Low-incidence countries have several challenges in common, including decreasing political commitment to tuberculosis elimination, reduced awareness of tuberculosis among the general public, and diminishing clinical expertise, which have accompanied declining tuberculosis incidence. Additionally, tuberculosis epidemiology in low-incidence countries tends to be characterised by a low rate of transmission in the general population, with most cases of tuberculosis resulting from reactivation of latent tuberculosis infection, which has major implications for the approach to tuberculosis elimination. Finally, although usually accounting for a small minority of cases in many low-incidence countries, homeless people and other hard-to-reach populations including migrants are ongoing sources of local tuberculosis transmission that generate new cases of latent tuberculosis infection.3
The USA is a low-incidence country with an annual tuberculosis incidence of 30 per 1 million people.4 Although substantial progress has been made in reducing the burden of tuberculosis, the USA faces the same challenges of other low-incidence countries. In this Personal View we highlight the experience of the USA in tuberculosis elimination efforts to date and explore the next steps towards elimination. Although there are unique elements that affect the US tuberculosis elimination effort (eg, the health-care system), the history of tuberculosis in the USA, remaining challenges, and proposed solutions, might have substantial relevance to other low-incidence countries.
The initial plan to eliminate tuberculosis in the USA
In 1989, tuberculosis was thought to be retreating into geographically and demographically defined pockets in the USA. Better diagnostic, treatment, and prevention methods were becoming available, and new computer and telecommunications technology enhanced the capacity of clinicians and public health systems to apply them. Because of these developments, the Advisory Committee for Elimination of Tuberculosis (ACET) recommended to the US Centers for Disease Control and Prevention (CDC) specific actions that would achieve tuberculosis elimination (defined as an incidence of less than one tuberculosis case per 1 million people) by 2010.5 However, in 2015, the US tuberculosis case rate was 30-times the elimination threshold, and the number of tuberculosis cases reported was higher than the previous year for the first time in over two decades.4 These indicators of limited progress raise two questions: why did the US tuberculosis elimination effort stall, and what needs to be done to accelerate progress toward the elimination of tuberculosis in the USA?
The resurgence and response of tuberculosis in the USA
When the ACET plan was published, the council’s assumption that tuberculosis was retreating into geographically and demographically defined pockets was inaccurate. Beginning in 1953, when systematic national tuberculosis cases counts first became available, tuberculosis cases steadily decreased from approximately 84 000 to 22 000 in 1985.6 However, from 1986, to 1992, tuberculosis cases increased annually, peaking at over 26 000 (figure 1). This resurgence in tuberculosis has been attributed to the onset of the HIV epidemic, transmission of tuberculosis, including multidrug-resistant tuberculosis, in health-care facilities and other congregate settings, deterioration of tuberculosis programme infrastructure, and increased immigration of people from countries with higher tuberculosis rates than the USA.7 In response, large increases in resources were provided at the national, state, and local levels.8,9 These resources allowed programmatic and laboratory improvements, such as widespread implementation of directly observed therapy, systematic contact investigations, infection control measures in congregate settings, and use of liquid culture, as well as research that contributed to availability of same-day nucleic acid amplification tests for tuberculosis, interferon-γ release assays (IGRAs), and short-course treatment for latent tuberculosis infection.8–12 Tuberculosis cases declined steadily from 1993 until the end of 2013, when 9421 tuberculosis cases were reported. This progress levelled off recently, with a 1.5% decrease in tuberculosis cases in 2014 (the smallest decrease in a decade), and a small increase in cases in 2015.4,6
Figure 1. Reported tuberculosis cases in the USA, 1982–2014.
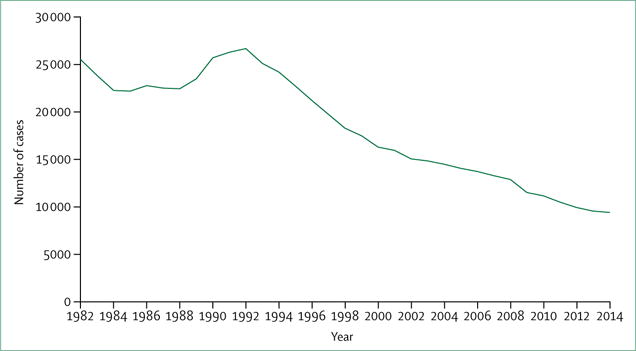
Data from US Centers for Disease Control and Prevention.6
A major shift in epidemiology accompanied the decrease in number of annual US tuberculosis cases. In 1993, 69% of tuberculosis cases occurred in people born in the USA, with 29% occurring in foreign-born people (the remaining [<2%] with unknown country of birth).6 By 2014, this had reversed (34% of cases in US-born people and 66% in foreign-born people) (figure 2).6 Because foreign-born people have a much higher likelihood of having been infected with Mycobacterium tuberculosis in their birth country than in the USA (where tuberculosis exposure is less probable in view of the low incidence), this change in epidemiology has substantial implications for tuberculosis elimination in the USA.
Figure 2. Trends in tuberculosis cases in foreign-born people in the USA, 1993–2014.
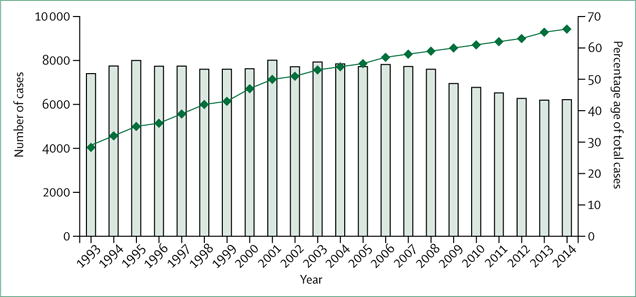
Data from US Centers for Disease Control and Prevention.6
Ending historical neglect: tuberculosis elimination revisited
A decade after the ACET plan was published,5 with tuberculosis cases declining again, the Institute of Medicine (IOM) was commissioned to review the state of tuberculosis elimination efforts in the USA. IOM published its findings in 2000, in the report entitled Ending Neglect: the Elimination of Tuberculosis in the United States.7 This report called for aggressive and decisive action to reinvigorate tuberculosis elimination efforts on the basis of five strategies (referred to as goals in the IOM report): (1) maintain existing tuberculosis control efforts through case detection, case management, and infection control; (2) increase focus on latent tuberculosis infection to accelerate the decline in tuberculosis; (3) research new diagnostics and treatments; (4) increase US involvement in efforts to improve tuberculosis control globally; and (5) mobilise support for tuberculosis elimination and monitor progress toward that goal. By 2010, the target year for tuberculosis elimination in the ACET plan, the tuberculosis rate in the USA had decreased by more than 50% from 1989 to 36 per 1 million people.6 However, the case rate was still 36-times the elimination target, despite 18 consecutive years of annual decline. What went wrong?
Implementation of the five IOM strategies met with mixed success in advancing the cause of tuberculosis elimination. With regard to the activities under strategy 1 (maintain tuberculosis control), high treatment completion rates have been achieved for people with tuberculosis, and there have been substantial improvements in infection control, especially in health-care settings.6,13 Conversely, delays in diagnosis still occur, and most programmes do not meet targets for testing and treatment initiation and completion for latent tuberculosis infection in contact investigations.14–16 For strategy 2, data for testing and treatment for latent tuberculosis infection indicate that whereas treatment is initiated in several hundred thousand people with latent tuberculosis infection per year, the infection is present in up to 13 million people.17,18 New tools developed under strategy 3, such as IGRAs and nucleic acid amplification tests, have been mostly helpful, but are incremental rather than transformational improvements.19,20 With regard to strategy 4, the USA has been substantially engaged in global tuberculosis efforts, but global tuberculosis incidence has only recently begun to decrease at a modest rate of approximately 2% per year.21–23 Finally, mobilising support for tuberculosis elimination (strategy 5) has been challenging because as tuberculosis cases continue to decline, addressing tuberculosis can seem less urgent for policy makers and the public compared with diseases that are increasing in prevalence.24
Looking forward, the area with the greatest potential for advancing tuberculosis elimination appears to be strategy 2. Both modelling and surveillance data about the origin of most tuberculosis cases in the USA indicate that the greatest reduction in future tuberculosis cases will result from expanded testing and treatment of latent tuberculosis infection. Furthermore, developments in science and policy suggest there are new opportunities to increase the amount and effectiveness of latent tuberculosis infection testing and treatment.
Latent tuberculosis infection: the final frontier of tuberculosis elimination
Both epidemiological data and modelling underscore the necessity of addressing latent tuberculosis infection if progress is to be made in eliminating tuberculosis in the USA. A 2015 analysis25 of recent tuberculosis transmission within the USA indicated that more than 85% of tuberculosis cases originated from reactivation of latent tuberculosis infection. This analysis is consistent with two other pieces of data: most (about 70%) tuberculosis cases occur in foreign-born people, and most (about 70%) tuberculosis cases in foreign-born people occur at least 2 years after entry to the USA.6,26 Because the USA has a very low tuberculosis incidence, it is much more likely that tuberculosis infection was acquired outside of the USA for foreign-born people, especially because the vast majority (about 95%) of these cases occur in people emigrating from countries with much higher tuberculosis rates than the USA.6,26
A revised approach to screening of US-bound immigrants and refugees, which has added routine culture and drug-susceptibility testing to smear microscopy, has been successful in detecting and treating more tuberculosis cases in these populations than before, thereby reducing importation of prevalent tuberculosis.27 However, the programme does not include routine latent tuberculosis infection testing and treatment, and therefore does not affect importation of latent tuberculosis infection. Additionally, the screening programme is limited to immigrants who have been granted permanent resident visas and refugees, and does not include student or work visa holders.
Modelling further supports the importance of addressing latent tuberculosis infection. In a mathematical tuberculosis transmission model, it was found that major reductions in US tuberculosis incidence, including reaching elimination in the US-born population, could be achieved if latent tuberculosis infection treatment was substantially increased (four-times).28 Although the foreign-born tuberculosis case rate could also be cut with increased treatment for latent tuberculosis infection, it would plateau just below 50 per 1 million people. One cause of this plateau is the continuing importation of latent tuberculosis infection in new immigrants. The case rate in foreign-born people could be cut much further with the overall case rate approaching, but not quite reaching, elimination if the prevalence of latent tuberculosis infection in foreign-born arrivals is reduced to 25% of the baseline prevalence estimated in 2000 (18.7%)—ie, 4.7%.28 This finding supports the strong need for strategy 4, US engagement in global tuberculosis activities. By helping to reduce global tuberculosis prevalence and consequently reducing global latent tuberculosis infection, the USA would be contributing indirectly to its own domestic tuberculosis elimination effort.
The latent tuberculosis infection prevention cascade
Analogous to the HIV care continuum (or treatment cascade) from infection to viral suppression, we can describe a latent tuberculosis infection prevention cascade (figure 3). The sequence of steps is: identify population to be tested, test with IGRA or tuberculin skin test (TST, including evaluation to exclude tuberculosis if test is positive), initiate treatment in people who test positive for latent tuberculosis infection, and complete treatment.
Figure 3. Latent tuberculosis infection prevention cascade using the example of contact investigation.
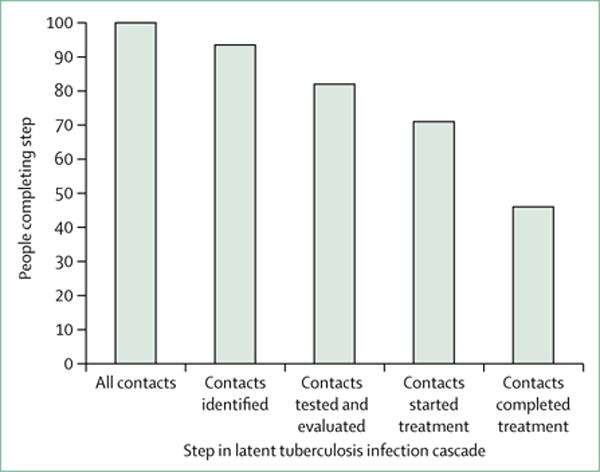
The cascade consists of multiple steps: identifying the population at risk, testing the population at risk for latent tuberculosis infection, and evaluating those with positive tests to exclude tuberculosis; initiating treatment in those with latent tuberculosis infection, and completing treatment for those who start. People can fail to complete any of the steps, and the effect is multiplicative. In this example, it is assumed that 93% of contacts have been identified and 82% of those have a complete evaluation. Of the contacts with latent tuberculosis infection (21%), 71% start treatment, and 46% of those who start treatment complete it. Thus, only 33% of contacts with latent tuberculosis infection complete treatment.16
People can fail to complete any of these steps and the effect is multiplicative, as shown in an analysis16 of US contact investigations (figure 3). There are several factors that make testing and treatment of latent tuberculosis infection challenging. To begin with, the reservoir of people with latent tuberculosis infection (up to 13 million cases per year) is vast compared with the number of new cases of tuberculosis (fewer than 10 000 cases per year).6,17 It has been estimated that between 300 000 and 400 000 people with latent tuberculosis infection are treated in the USA each year, which is well below what would be needed to have a substantial impact.17,18
The tests for latent tuberculosis infection, TST and IGRA, are poorly predictive of who will eventually progress to tuberculosis. Only 5–10% of people with latent tuberculosis infection will ultimately progress to tuberculosis when the TST result is positive.29 This predictive power appears to improve slightly, to approximately 13%, when IGRA is used.30 With a relatively small chance of becoming ill, it can be difficult to convince a patient to take medication for months for a condition that is asymptomatic at the time of detection. For the TST, this problem with acceptance of treatment is further complicated by the cross-reaction that can occur in people vaccinated with BCG.20 Physicians and patients who are aware of this possible cross-reaction could attribute a positive test result to BCG vaccination rather than to latent tuberculosis infection.
Finally, treatment with isoniazid for 6–12 months, which has been the mainstay for latent tuberculosis infection treatment for decades, has low completion rates.31 Additionally, this regimen’s most concerning adverse effect (hepatotoxicity), although relatively rare and manageable, can be severe if not recognised promptly.31
Opportunities for testing and treatment of latent tuberculosis infection
Although systematically tackling latent tuberculosis infection might seem challenging, there are new opportunities that make the undertaking more feasible. Although, overall, IGRAs might be an incremental improvement over the TST, they have substantial advantages in some key populations at risk for latent tuberculosis infection. There is no cross-reaction with the BCG vaccine. When BCG-vaccinated populations are tested, they consistently have a lower percentage of IGRA-positive test results compared with TST, which has been attributed to lack of false-positive IGRA test results resulting from the BCG.20,30 Another advantage of IGRAs is that they only require one patient visit as opposed to the TST, which requires two visits (one for placement and one for reading). In certain populations (eg, homeless people), a substantial proportion of people might not return for the TST reading, requiring a repeat test.20
Better treatment regimens have also become available. 3 months of once-weekly isoniazid and rifapentine (3HP) or 4 months of daily rifampicin have been shown to have higher completion rates with less hepatotoxicity compared with 9 months of isoniazid alone.32,33 A disadvantage of the 3HP regimen has been that the major efficacy study was done using directly observed therapy, and therefore it is initially recommended to be used with directly observed therapy.12 Even with the added cost of directly observed therapy, the regimen is still cost-effective.34 Additionally, preliminary data from a randomised clinical trial indicate that self-administered 3HP is safe, with completion rates still superior to those historically achieved with 9 months of isoniazid alone, which suggests that the regimen is highly effective without the added cost of directly observed therapy.35 In the interim, use of video directly observed therapy could be considered to decrease costs.36 To increase both acceptance and completion of testing and treatment, other interventions that can be considered include incentives and enablers, culturally specific education or counselling, and increased access through expanded clinic hours.37,38 While not currently used in the USA, 3 months of daily isoniazid and rifampicin is another short-course regimen that has been used successfully in other countries.39
A potential approach to expansion of latent tuberculosis infection testing and treatment
In view of the large reservoir of latent tuberculosis infection, making progress will require a major initiative consisting of five parts: a surveillance system or registry of latent tuberculosis infection to monitor progress, scale-up of targeted latent tuberculosis infection testing using IGRA in at-risk populations with a particular focus on foreign-born people from countries with high incidence of tuberculosis, scale-up of short-course treatment regimens, engagement of affected communities and medical providers who serve those communities, and increased public health staffing for implementation and oversight.
Currently, there is a comprehensive national system for surveillance of tuberculosis, but only sporadic, mostly state-based surveillance of latent tuberculosis infection. The only national reporting related to latent tuberculosis infection is for contact investigation, and this system is limited to aggregate (rather than individual line-listed) reporting for latent tuberculosis infection testing and treatment.16 To adequately monitor progress in addressing latent tuberculosis infection, a more complete, individual case-based registry or surveillance system is needed.
Though the extent of testing for latent tuberculosis infection in the USA is unknown, the relatively limited treatment of latent tuberculosis infection we describe suggests that testing of high-risk groups is also likely to be relatively low. Major expansion of testing, especially in foreign-born people who account for nearly 70% of tuberculosis cases in the United States and who have a latent tuberculosis infection prevalence as much as 13-times higher than for US-born people, is required to accelerate tuberculosis elimination.6,17,28 To have a major effect, a very broad segment of this population would need to be included in testing, because tuberculosis cases are not concentrated in a particular subgroup (recent vs remote immigration, one or two specific countries, method of entry [eg, immigrants who have become permanent residents vs refugee]).6 It might be more feasible to initially concentrate on immigrants with the highest risk for tuberculosis (eg, refugees, recent immigrants, or immigrants with comorbid conditions that increase the risk of tuberculosis reactivation), but ultimately expansion to include most people from countries of medium and high tuberculosis burden, regardless of other factors, would probably be necessary to substantially reduce tuberculosis cases.26,40 One approach to expand testing would be to strengthen the link between overseas tuberculosis screening of immigrants and refugees (as discussed previously) and latent tuberculosis infection testing and treatment. Immigrants and refugees identified as needing further evaluation for tuberculosis, or as having inactive tuberculosis, have electronic notifications sent to local health departments.41 It is probable that a substantial portion of such people have latent tuberculosis infection and could be tested and treated during a follow-up evaluation in the USA. However, 30–40% of immigrants and refugees do not receive a complete evaluation in the USA, and there is no systematic information about latent tuberculosis infection testing and treatment in this group.42
Concurrent with major expansion of testing in high-risk groups, testing in low-risk groups, which is sometimes required by antiquated regulations, should cease. In addition to protecting individuals from the risk of unnecessary treatment because of false–positive test results, the resources used for testing low-risk groups would be better applied to the expansion of testing in high-risk groups. Expanded testing will only have an effect if it is associated with an expansion in effective treatment. Because of shorter duration, reduced hepatotoxicity, and increased completion rates, use of 3HP or 4 months of rifampicin, instead of 9 months of isoniazid, is preferable.
One of the major limitations of latent tuberculosis infection testing and treatment is that it appears to be done mostly by health departments.18 To accomplish substantial expansion, health departments will need to engage primary-care providers and communities with high latent tuberculosis infection prevalence, so that latent tuberculosis infection testing and treatment will be offered and accepted in high-risk populations. However, even if most of the testing and treatment is done by primary-care providers, health departments will still require additional staff and resources to provide leadership, guidance, and oversight. The UK provides an example of a collaborative latent tuberculosis infection testing programme focused on the primary care setting. Public Health England and the National Health Service jointly recommend latent tuberculosis infection testing for people aged 16 to 35 years, who entered the UK from a high incidence country (≥1500 per 1 million people, or sub-Saharan Africa) within the last 5 years and have been previously living in that high incidence country for 6 months or longer.43 Furthermore, these agencies indicate that the optimum setting for latent tuberculosis infection testing of new entrants is primary care. With regard to the economic implications of expanded latent tuberculosis infection testing in immigrants, there have been several systematic reviews and meta-analyses that have found IGRA-based testing of immigrants to be cost-effective.44–46
Research needs related to latent tuberculosis infection
As stated in Ending Neglect,7 a key step in advancing towards the ultimate elimination of tuberculosis is the development of “new diagnostic tests, particularly for diagnosis of infection”. A test that detects latent tuberculosis infection that is more likely to progress to tuberculosis or a serological marker of reactivation risk would dramatically increase the efficiency of latent tuberculosis infection testing and treatment. Although newer latent tuberculosis infection regimens are likely to improve the effectiveness of latent tuberculosis infection treatment, shortening the regimen even more, perhaps to 4–6 weeks, would be highly beneficial. An appealing alternative would be an effective vaccine that could prevent progression from latent tuberculosis infection to tuberculosis, but this does not seem likely to appear in the near future. Operational and economic evaluation research is also needed. Specific research areas include the feasibility of pre-entry testing and treatment for latent tuberculosis infection for immigrants to the USA, reducing tuberculosis risk in people with HIV and other conditions, ways to improve access to care, assessing different approaches to US investment in global tuberculosis control, and determining the cost-effectiveness of these various interventions and approaches.
Conclusion
The goal of tuberculosis elimination in the USA was first proposed in 1989, with a target date of 2010. 5 years after this target date, the tuberculosis case rate remained 30-times the elimination threshold. Tuberculosis epidemiology has changed substantially since the early 1990s, most notably with the proportion of foreign-born tuberculosis cases increasing from 29% in 1993 to 66% in 2014. Molecular epidemiological analyses have shown that more than 85% of tuberculosis cases are the result of reactivation of latent tuberculosis infection, rather than from recent transmission within the USA. The steady decline in tuberculosis cases has levelled off after two decades as the limits of a strategy that is primarily focused on detection and treatment of tuberculosis and implementation of infection control precautions, which are most effective at preventing further transmission, have been reached. It is crucial that high treatment completion rates for tuberculosis and strong implementation of infection control measures be maintained to prevent a tuberculosis resurgence, such as that which occurred in the 1980s and early 1990s. However, the data cited above suggest that further advances toward tuberculosis elimination will require a major effort to better address latent tuberculosis infection in people at high risk of reactivated tuberculosis. This effort should consist of a surveillance system or registry to monitor progress, scale-up of targeted latent tuberculosis infection testing in at-risk populations, scale-up of short-course treatment regimens, engagement of affected communities and medical providers who serve those communities, and increased public health staffing for implementation and oversight. Such an effort would be greatly facilitated by the development of new tools, such as tests that better indicate reactivation risk and even shorter latent tuberculosis infection treatment regimens.
Search strategy and selection criteria.
We searched PubMed using the terms “latent tuberculosis infection” or “tuberculosis elimination” for manuscripts published from Jan 1, 1989, to April 1, 2016. The results were restricted to papers published in English. We included selected publications that provided relevant information on latent tuberculosis infection with regard to its epidemiology, diagnosis, and treatment. We included selected publications that provided relevant information about tuberculosis elimination, with regard to proposed strategies and their implementation, particularly in the USA and other countries with low tuberculosis incidence.
Footnotes
Contributors
PAL contributed to the conception and design of the Personal View, the review and interpretation of data cited in the manuscript, wrote the initial draft of the Personal View, and was involved in revising the Personal View. JHM contributed to the conception and design of the Personal View, the review and interpretation of data cited in the Personal View, and provided important revisions and content. Both authors provided final approval of the version to be published.
Declaration of interests
We declare no competing interests.
References
- 1.Uplekar M, Weil D, Lonnroth K, et al. WHO’s new End TB Strategy. Lancet. 2015;385:1799–801. doi: 10.1016/S0140-6736(15)60570-0. [DOI] [PubMed] [Google Scholar]
- 2.Lonnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015;45:928–52. doi: 10.1183/09031936.00214014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad MB, Mitruka K, Oeltmann JE, Johns EB, Navin TR. Characteristics of tuberculosis cases that started outbreaks in the United States, 2002–2011. Emerg Infect Dis. 2015;21:508–10. doi: 10.3201/eid2103.141475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence—United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65:273–78. doi: 10.15585/mmwr.mm6511a2. [DOI] [PubMed] [Google Scholar]
- 5.US Centers for Disease Control and Prevention. A strategic plan for the elimination of tuberculosis in the United States. MMWR Morb Mortal Wkly Rep. 1989;38:269–72. [PubMed] [Google Scholar]
- 6.US Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2015. [Google Scholar]
- 7.Institutue of Medicine. Ending neglect: the elimination of tuberculosis in the United States. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 8.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333:229–33. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 9.Castro KG, Marks SM, Chen MP, et al. Estimating tuberculosis cases and their economic costs averted in the United States over the past two decades. Int J Tuberc Lung Dis. 2016;20:926–33. doi: 10.5588/ijtld.15.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Centers for Disease Control and Prevention. Nucleic acid amplification tests for tuberculosis. MMWR Morb Mortal Wkly Rep. 1996;45:950–52. [PubMed] [Google Scholar]
- 11.Mazurek GH, Villarino ME. Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep. 2003;52:15–18. [PubMed] [Google Scholar]
- 12.US Centers for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650–53. [PubMed] [Google Scholar]
- 13.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54:1–141. [PubMed] [Google Scholar]
- 14.Bloss E, Newbill K, Peto H, et al. Challenges and opportunities in a tuberculosis outbreak investigation in southern Mississippi, 2005–2007. South Med J. 2011;104:731–35. doi: 10.1097/SMJ.0b0.3e318232679e. [DOI] [PubMed] [Google Scholar]
- 15.Golub JE, Bur S, Cronin WA, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis. 2006;10:24–30. [PubMed] [Google Scholar]
- 16.Young KH, Ehman M, Reves R, et al. Tuberculosis contact investigations—United States, 2003–2012. MMWR Morb Mortal Wkly Rep. 2016;64:1369–74. doi: 10.15585/mmwr.mm6450a1. [DOI] [PubMed] [Google Scholar]
- 17.Miramontes R, Hill AN, Yelk Woodruff RS, et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS One. 2015;10:e0140881. doi: 10.1371/journal.pone.0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterling TR, Bethel J, Goldberg S, Weinfurter P, Yun L, Horsburgh CR. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173:927–31. doi: 10.1164/rccm.200510-1563OC. [DOI] [PubMed] [Google Scholar]
- 19.US Centers for Disease Control and Prevention. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep. 2009;58:7–10. [PubMed] [Google Scholar]
- 20.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 21.WHO. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 22.US Agency for International Development. Accelerating progress in the global effort against tuberculosis. Washington, DC: US Agency for International Development; 2014. [Google Scholar]
- 23.The Global Fund Government Donors. The Global Fund. 2016 http://www.theglobalfund.org/en/government/ (accessed April 7, 2017).
- 24.Stop TB USA Tuberculosis Elimination Plan Committee. A call for action on the tuberculosis elimination plan for the United States. Atlanta, GA: Stop TB USA; 2010. [Google Scholar]
- 25.France AM, Grant J, Kammerer JS, Navin TR. A field-validated approach using surveillance and genotyping data to estimate tuberculosis attributable to recent transmission in the United States. Am J Epidemiol. 2015;182:799–807. doi: 10.1093/aje/kwv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cain KP, Benoit SR, Winston CA, Mac Kenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300:405–12. doi: 10.1001/jama.300.4.405. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Posey DL, Cetron MS, Painter JA. Effect of a culture-based screening algorithm on tuberculosis incidence in immigrants and refugees bound for the United States: a population-based cross-sectional study. Ann Intern Med. 2015;162:420–28. doi: 10.7326/M14-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill AN, Becerra J, Castro KG. Modelling tuberculosis trends in the USA. Epidemiol Infect. 2012;140:1862–72. doi: 10.1017/S095026881100286X. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society, US Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 30.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-gamma release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med. 2011;183:88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 31.Lobue P, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology. 2010;15:603–22. doi: 10.1111/j.1440-1843.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- 32.Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–66. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 33.Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170:445–49. doi: 10.1164/rccm.200404-478OC. [DOI] [PubMed] [Google Scholar]
- 34.Shepardson D, Mac Kenzie WR. Update on cost-effectiveness of a 12-dose regimen for latent tuberculous infection at new rifapentine prices. Int J Tuberc Lung Dis. 2014;18:751. doi: 10.5588/ijtld.14.0052. [DOI] [PubMed] [Google Scholar]
- 35.Belknap R, Borisov A, Holland D, et al. Adherence to once-weekly self-administered INH and rifapentine for latent TB: iAdhere (abstract). Conference on Retroviruses and Opportunistic Infections; Feb 23–26, 2015.Seattle, WA: [Google Scholar]
- 36.Story A, Garfein RS, Hayward A, et al. Monitoring therapy compliance of tuberculosis patients by using video-enabled electronic devices. Emerg Infect Dis. 2016;22:538–40. doi: 10.3201/eid2203.151620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malotte CK, Hollingshead JR, Larro M. Incentives vs outreach workers for latent tuberculosis treatment in drug users. Am J Prev Med. 2001;20:103–07. doi: 10.1016/s0749-3797(00)00283-x. [DOI] [PubMed] [Google Scholar]
- 38.Ailinger RL, Martyn D, Lasus H, Lima Garcia N. The effect of a cultural intervention on adherence to latent tuberculosis infection therapy in Latino immigrants. Public Health Nurs. 2010;27:115–20. doi: 10.1111/j.1525-1446.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 39.Bright-Thomas R, Nandwani S, Smith J, Morris JA, Ormerod LP. Effectiveness of 3 months of rifampicin and isoniazid chemoprophylaxis for the treatment of latent tuberculosis infection in children. Arch Dis Child. 2010;95:600–02. doi: 10.1136/adc.2010.182600. [DOI] [PubMed] [Google Scholar]
- 40.Hadzibegovic DS, Maloney SA, Cookson ST, Oladele A. Determining TB rates and TB case burden for refugees. Int J Tuberc Lung Dis. 2005;9:409–14. [PubMed] [Google Scholar]
- 41.Lee D, Philen R, Wang Z, et al. Disease surveillance among newly arriving refugees and immigrants—Electronic Disease Notification System, United States, 2009. MMWR Surveill Summ. 2013;62:1–20. [PubMed] [Google Scholar]
- 42.Liu Y, Weinberg MS, Ortega LS, Painter JA, Maloney SA. Overseas screening for tuberculosis in US-bound immigrants and refugees. N Engl J Med. 2009;360:2406–15. doi: 10.1056/NEJMoa0809497. [DOI] [PubMed] [Google Scholar]
- 43.Public Health England and the National Health Service. Latent TB testing and treatment for migrants: a practical guide for commissioners and practitioners. London: Public Health England; 2015. [Google Scholar]
- 44.Campbell JR, Sasitharan T, Marra F. A systematic review of studies evaluating the cost utility of screening high-risk populations for latent tuberculosis infection. Appl Health Econ Health Policy. 2015;13:325–40. doi: 10.1007/s40258-015-0183-4. [DOI] [PubMed] [Google Scholar]
- 45.Zammarchi L, Casadei G, Strohmeyer M, et al. A scoping review of cost-effectiveness of screening and treatment for latent tubercolosis infection in migrants from high-incidence countries. BMC Health Serv Res. 2015;15:412. doi: 10.1186/s12913-015-1045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nienhaus A, Schablon A, Costa JT, Diel R. Systematic review of cost and cost-effectiveness of different TB-screening strategies. BMC Health Serv Res. 2011;11:247. doi: 10.1186/1472-6963-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]


