Abstract
Background
The NICHD Stillbirth Collaborative Research Network (SCRN) previously demonstrated an association between stillbirth and maternal marijuana use as defined by the presence of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid (THC) in the umbilical cord homogenate. However, the relationship between marijuana use and perinatal complications in live births is uncertain.
Objective
Our aim was to examine if maternal marijuana use is associated with increased odds of adverse pregnancy outcomes and neonatal morbidity among liveborn controls in the SCRN cohort.
Study Design
Secondary analysis of singleton, liveborn controls in the SCRN dataset. Marijuana use was measured by self-report and/or the presence of THC in umbilical cord homogenate. Tobacco use was measured by self-report and/or presence of any cotinine in maternal serum. Adverse pregnancy outcome was a composite of small for gestational age (SGA), spontaneous preterm birth resulting from preterm labor with or without intact membranes (SPTB), and hypertensive disorders of pregnancy (HTN). Neonatal morbidity included neonatal intensive care unit (NICU) admission and composite neonatal morbidity (pulmonary morbidity, necrotizing enterocolitis, seizures, retinopathy of prematurity, infection morbidity, anemia requiring blood transfusion, neonatal surgery, hyperbilirubinemia, neurological morbidity or death prior to hospital discharge). Effect of maternal marijuana use on the probability of an adverse outcome was estimated using weighted methodology to account for over-sampling in the original study. THC cord homogenate analysis was performed in the subset of women for whom biospecimens were available. Comparisons using logistic modeling, chi-square, and t-tests were weighted to account for oversampling of preterm births and non-Hispanic blacks. Results are reported as weighted percent and unweighted frequencies.
Results
Maternal marijuana use was identified in 2.7% (unweighted frequency 48/1610) of live births. Use was self-reported by 1.6% (34/1610) and detected by THC in cord homogenate for 1.9% (17/897), n=3 overlapping. Rate of tobacco use was 12.9% (217/1610), with 10.7% (167/1607) by self-report and 9.5% (141/1313) by serum cotinine. The composite adverse pregnancy outcome was not significantly increased in women with marijuana use compared to non-users (31.2% versus 21.2%, p=0.14).
After adjustment for tobacco, clinical and socioeconomic factors, marijuana use was not associated with the composite adverse pregnancy outcome (aOR 1.29, 95% CI 0.56–2.96). Similarly, among women with umbilical cord homogenate and serum cotinine data (n=765), marijuana use was not associated with adverse pregnancy outcomes (aOR 1.02, 95% CI 0.18–5.66).
NICU admission rates were not statistically different between groups (16.9% users versus 9.5% non-users, p=0.12). Composite neonatal morbidity or death was more frequent among neonates of mothers with marijuana use compared to non-users (14.1% versus 4.5%, p=0.002). In univariate comparisons, the components of the composite outcome that were more frequent in neonates of marijuana users were infection morbidity (9.8% versus 2.4%, p<0.001), and neurologic morbidity (1.4% versus 0.3%, p=0.002). After adjustment for tobacco, race and other illicit drug use, marijuana use was still associated with composite neonatal morbidity or death (aOR 3.11, 95% CI 1.40–6.91).
Conclusion
Maternal marijuana use was not associated with a composite of SGA, SPTB, or HTN. However, it was associated with an increased risk of neonatal morbidity.
Keywords: adverse pregnancy outcome, biological sampling, maternal marijuana use, THC, umbilical cord homogenate
Introduction
Legalization of marijuana in the United States has resulted in a resurgence of interest in its health effects. More women now report using marijuana either recreationally or to treat nausea and vomiting of pregnancy.1 Between 2002 and 2014, past-month use among pregnant women increased from 2.4% to 3.9%.2 With increasing use, there is an urgent need for high quality data regarding the health effects of marijuana in pregnancy in order to appropriately educate women and healthcare providers.
Previous studies on the association between marijuana use and perinatal outcomes have yielded mixed results,3–8 likely due to incomplete ascertainment of exposure without quantification of marijuana use, and without corroboration of use with biological sampling.3
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Stillbirth Collaborative Research Network (SCRN) recently demonstrated an association between maternal marijuana use and stillbirth.9 The SCRN utilized umbilical cord homogenate assays, allowing for detection of use from the second trimester onward. Our objective was to examine if maternal marijuana use is associated with increased odds of adverse pregnancy outcomes (small for gestational age (SGA), spontaneous preterm birth (SPTB), and hypertensive disorders of pregnancy (HTN)) and neonatal morbidity (neonatal intensive care unit (NICU) admission, composite neonatal morbidity and/or death) among liveborn controls in the SCRN cohort.
Materials and Methods
This was a SCRN secondary data analysis. The original study enrolled stillbirths in a geographically and racially diverse population. Liveborn controls between 20–31 weeks were oversampled at the time of the original study in order to provide numbers similar to those of stillbirths during each gestational week, and were therefore oversampled for adverse pregnancy outcomes resulting in preterm births. Prospective enrollment occurred from March 2006 through September 2008. Details regarding the methodology of the original study and selection of the liveborn controls have been previously published.10 The study was approved by the Institutional Review Boards of each participating institution.
For this analysis, all women with non-anomalous singleton live births ≥ 24 weeks were considered for inclusion. Women with missing obstetrical history data, and/or drug or tobacco use were excluded. The adverse pregnancy outcome was a composite of SGA, SPTB and HTN. SGA was defined as a birth-weight less than the 10%ile for gestational age.11 SPTB was defined as a preterm delivery (<37 weeks) resulting from spontaneous preterm labor with or without intact membranes. HTN included gestational hypertension and preeclampsia. Standard definitions of hypertensive disorders of pregnancy were utilized.10 Women without data available for any component of the composite adverse pregnancy outcome were excluded.
Neonatal morbidity outcomes included NICU admission, or a composite neonatal morbidity defined as any evidence of neonatal pulmonary morbidity (respiratory distress syndrome, persistent pulmonary hypertension, bronchopulmonary dysplasia, pulmonary hypoplasia), necrotizing enterocolitis, seizures, retinopathy of prematurity, infection morbidity (sepsis - clinical or blood culture confirmed, pneumonia, or bacterial meningitis), anemia requiring blood transfusion, neonatal surgery, hyperbilirubinemia, neurological morbidity (any grade of intraventricular hemorrhage or periventricular leukomalacia) or neonatal death prior to discharge. Standard neonatal morbidity definitions were utilized.12
Our primary exposure was maternal marijuana use as measured by self-report and/or the presence of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid (THC) in umbilical cord homogenate. Self-report of any drug use was obtained through a standardized maternal interview within 4 weeks of the delivery hospitalization and any use during pregnancy was obtained via chart abstraction by the research team.
Drug assays of umbilical cord segments occurred at the United States Drug Testing Laboratories, Inc (USDTL). A 3.5 gram segment of umbilical cord was drained of blood, rinsed with saline and dried. Specimens were frozen at −80° C in a 15 mL cryovial until the time of drug assay (THC is stable when stored at temperatures below −20° C).13 The cord was homogenized and drug assays were performed initially by enzyme-linked immunosorbent analysis (ELISA) for cannabinoids, amphetamine, methamphetamine, cocaine, meperidine, and hydrocodone. Positive results by ELISA were confirmed using mass spectrometry.13 The mass spectrometry methodology was a GC-GC/MS assay with Deans switch methodology and negative chemical ionization detection mass spectrometry for THC similar to that described for USDTL hair assays.14 In comparison to meconium drug assays, umbilical cord homogenate sampling detects maternal marijuana use in term infants from as early as 20 weeks gestation.13,15 The placenta and umbilical cord were not always collected at delivery so not all participants had samples available. Cord collection and processing details were previously published.16 For this analysis, illicit drug use was defined as reported use of heroin, cocaine (street name “crack”), amphetamines including crystal methamphetamine, inhalants, 3,4-methylenedioxymethamphetamine (street name “ecstasy”), hallucinogens, or a drug assay positive for one of these substances.
Tobacco use is an important confounder, and was measured by self-report and/or detectable maternal serum cotinine. Self-reported tobacco was defined as reporting 1 or more cigarettes every day in any trimester or any tobacco use after diagnosis of pregnancy. Serum samples were collected at delivery admission. Specimens were centrifuged at 1,300g for 15 minutes, then aliquoted into cryovials and stored at −80° C until the time of assay. Cotinine assays were performed using liquid chromotography.17 For our primary analysis, any cotinine in the maternal serum was considered indicative of tobacco use. In sensitivity analysis, cotinine was defined with three levels of exposure: none (undetectable), passive (<3ng/mL), and presumed smoker (≥3ng/mL) based on previously established cut-offs.9
We used chi square and t test as appropriate to compare demographic characteristics between women who used marijuana to non-users. Groups were also compared for frequencies of the adverse composite outcome and each individual component. All calculated comparisons utilized weightings that reflected the original study design of oversampling at early gestational ages and for non-Hispanic black race. The methodology for weighting the sample has been published previously.10
The effect of maternal marijuana use on the probability of the composite adverse pregnancy outcome was estimated using a logistic regression model. Race, obstetrical history (categorized as nulliparous, parous with history of term births only, parous with history of preterm birth <37 weeks), tobacco use, illicit drug use, and body mass index (BMI) were determined to be clinically important covariates and were considered in multivariable modeling. In addition, socioeconomic factors that differed between groups in univariable analysis and the interaction between marijuana and tobacco were considered for inclusion. A final model was selected through sequential, backwards elimination of covariates with p>0.05. A second regression model of the composite adverse pregnancy outcome was estimated among the subset of women for whom biospecimens were available.
The effect of maternal marijuana use on the probability of the composite neonatal morbidity was estimated using a logistic regression model. Race, tobacco use, illicit drug use, and BMI were determined to be clinically important covariates and were considered in multivariable modeling. In addition, socioeconomic factors that differed between groups in univariable analysis and the interaction between marijuana and tobacco were considered for inclusion. A final model was selected through sequential, backwards elimination of covariates with p>0.05. A second model of composite neonatal morbidity among the subset of women with biospecimens was not estimated given the low frequency of neonatal morbidity among women with biospecimens available.
P < 0.05 was considered statistically significant. All analyses were completed in SAS 9.4 (specifically proc SurveyLogistic for modeling), with graphics created using Graphpad Prism v6.03.
Results
There were 1,996 liveborn controls in the data set. Three-hundred-eighty-six women were excluded (n=77 missing component of adverse pregnancy outcome, n=63 missing BMI, n=125 multiple gestation, n=74 missing drug use variable, n=137 births prior to 24 weeks gestational age, and n=14 congenital anomalies). Thus, 1,610 liveborn controls were included in the final cohort for analysis; 897 had umbilical cord tissue available.
Maternal marijuana use was identified in 2.7% (unweighted frequency 48/1610) of births. Marijuana use was self-reported by 1.6% (34/1610) and detected by THC in cord homogenate for 1.9% (17/897), n=3 overlapping. Overall agreement between self-reported marijuana use and umbilical cord homogenate results was negligible among women with biological sampling results available (kappa 0.0575, 95% CI −0.056–0.172). Among women with a positive cord homogenate for THC, 6.7% self-reported marijuana use. Among women self-reporting marijuana use, 8.0% tested positive via cord homogenate.
The rate of any tobacco use was 12.9% (217/1610), with 10.7% (167/1607) by self-report and 9.5% (141/1313) by maternal serum cotinine. Overall agreement between self-reported tobacco use and a positive maternal serum cotinine was moderate (kappa 0.6417, 95% CI (0.5645, 0.7188). Among women with a positive maternal serum cotinine, 72% self-reported tobacco use. Among women self-reporting tobacco use, 63% tested positive via maternal serum cotinine.
Women who used marijuana were more likely to use tobacco, use other illicit drugs (self-reported or cord homogenate-detected), be enrolled in Women, Infants and Children Supplemental Nutrition Program (WIC), and have a 12th grade or less education level when compared to non-users. Age, ethnicity, BMI, and obstetrical history did not differ by marijuana use (Table 1).
Table 1.
Demographics of Women who Use Marijuana Compared to Non-Users
| Maternal Characteristic |
Categorization | Any Marijuana Use (N=48) |
No Marijuana Use (N=1562) |
P value |
|---|---|---|---|---|
| Maternal age, years | <18 | 6.9 | 2.8 | 0.10 |
| 18–34 | 89.6 | 83.4 | ||
| ≥ 35 | 3.6 | 13.9 | ||
| Race/ethnicity | White, Multi-race or Other | 57.6 | 52.7 | 0.09 |
| Black | 19.9 | 11.1 | ||
| Hispanic | 22.5 | 36.2 | ||
| Body mass index, kg/m2 | <25 | 53.2 | 53.9 | 0.99 |
| 25-<30 | 23.3 | 23.2 | ||
| ≥ 30 | 23.5 | 22.9 | ||
| Tobacco use | Yes | 57.5 | 11.7 | <0.001 |
| Obstetrical history | Parous – Previous preterm birth | 12.0 | 9.4 | 0.86 |
| Parous – Previous term birth | 53.2 | 55.2 | ||
| Nulliparous | 34.8 | 35.5 | ||
| Enrolled in Women, Infants and Children (WIC) Supplemental Nutrition Program | Yes | 57.3 | 35.7 | 0.009 |
| Maternal education | 12th grade or less | 68.3 | 44.2 | 0.007 |
| Desired pregnancy | Yes | 94.4 | 94.6 | 0.93 |
| Self-reported illicit drug use* | Yes | 3.6 | 0.4 | <0.001 |
| Illicit drugs detected in cord homogenate* | Yes | 61.5 | 2.0 | <0.001 |
All results are reported as weighted percentages.
Self-reported illicit drug use includes cocaine (street name “crack”), heroin, amphetamines including crystal methamphetamine, inhalants, and 3,4-methylenedioxymethamphetamine (street name “ecstacy”). Cord homogenate results on subset of n=30 women who use marijuana, and n=867 non-users, includes all illicit drugs listed above. Maternal serum cotinine results were available on a subset of n=1313 women.
The composite adverse pregnancy outcome, and each component of the composite outcome, was present more frequently in women with marijuana use compared to non-users (Table 2). However, none of these differences were significant in univariable comparisons.
Table 2.
Adverse Pregnancy Outcomes in Women who Use Marijuana Compared to Non-Users
| Adverse Pregnancy Outcomes (Unweighted Sample Size) |
Any Marijuana Use (N=48) |
No Marijuana Use (N=1562) |
P value |
|---|---|---|---|
| Small for Gestational Age | 8.2 | 7.4 | 0.83 |
| Spontaneous Preterm Birth | 13.2 | 6.2 | 0.08 |
| Hypertensive Disorder | 14.0 | 9.0 | 0.28 |
| Composite Endpoint | 31.2 | 21.2 | 0.13 |
All results are reported as weighted percentages, p-value from weighted chi-square. The composite endpoint was defined by “any yes” or “all no”, as such some item non-response is present for individual components.
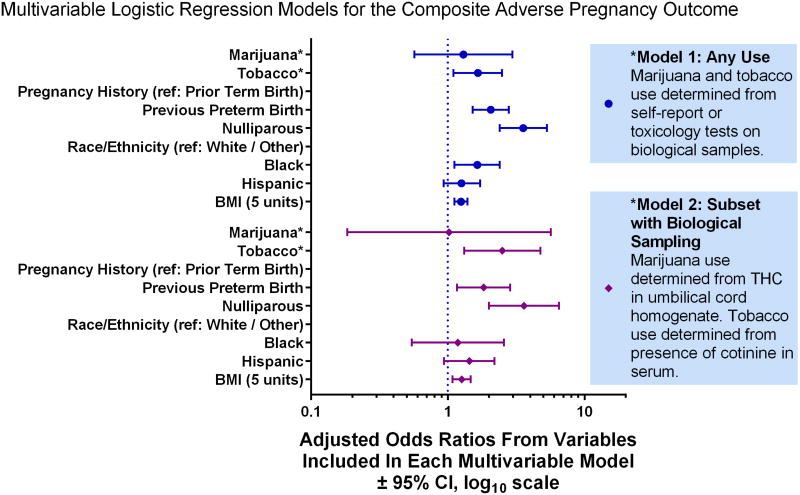
In the primary model any use of marijuana was included as the exposure (either self-report or THC by biological sampling). After adjustment for race, obstetrical history, and BMI, the association of marijuana use with the composite adverse pregnancy outcome was not significant (aOR 1.29, 95% CI 0.57–2.97), but that of tobacco use was (aOR 1.66, 95% CI 1.10–2.49) (Figure 1). The interaction between tobacco and marijuana use was non-significant (p>0.4).
Figure 1.
Multivariable modeling to estimate the association between maternal marijuana use and the composite adverse pregnancy outcome (spontaneous preterm birth, hypertensive disorders of pregnancy, and/or small for gestational age) while adjusting for tobacco use and other sociodemographic factors. Education, the interaction between tobacco and marijuana, illicit drug use, and participation in Women, Infants and Children Supplemental Nutrition Program were eliminated from the model as non-significant covariates.
In a second model, among women with umbilical cord homogenate and serum cotinine data (n=765), the association of marijuana use with the composite adverse pregnancy outcome remained non-significant (aOR 1.02, 95% CI 0.18–5.66), while the effect of tobacco use was larger in magnitude (aOR 2.50, 95% CI 1.32–4.75) in comparison to the primary model (Figure 1). Illicit drug use was not associated with adverse outcomes in either model. Since 59 women had cotinine levels less than 3 ng/mL and this may reflect only passive exposure, a sensitivity analysis with 3 categories of cotinine levels was performed. The odds ratio of marijuana use was similar in magnitude and significance (data not shown).
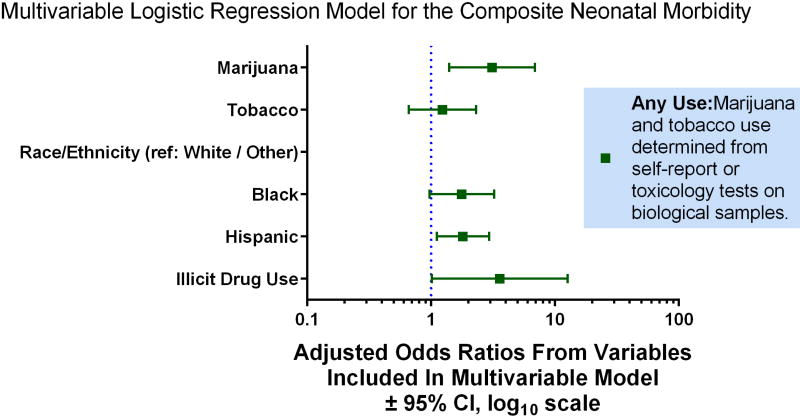
Women with any marijuana use (self-report or THC in cord homogenate) were compared to non-users for neonatal morbidity. Groups were similar with regard to NICU admission, and gestational age at delivery (38.4 ± 0.37 weeks users versus 38.8 ± 0.05 weeks non-users, p=0.277). However, composite neonatal morbidity or death was more frequent among neonates of mothers who used marijuana (Table 3). Upon further exploration of the components of composite neonatal morbidity, infection morbidity and neurological morbidity were significantly more frequent among women with marijuana use (Table 3). After adjustment for race and illicit drug use, marijuana use was associated with neonatal morbidity (aOR 3.11, 95% CI 1.40–6.91), and tobacco use was not (aOR 1.23, 95% CI 0.66–2.30) (Figure 2). The interaction between tobacco and marijuana use was non-significant (p>0.2).
Table 3.
Neonatal Morbidity in Women who Use Marijuana Compared to Non-Users
| Neonatal Outcome (Unweighted Sample Size) |
Any Marijuana Use (N=48) |
No Marijuana Use (N= 1562) |
P value |
|---|---|---|---|
| Neonatal intensive care unit admission | 16.9 | 9.5 | 0.12 |
| Preterm delivery (<37 weeks) | 17.3 | 9.0 | 0.07 |
| Composite neonatal morbidity or death | 14.1 | 4.5 | 0.002 |
| -Neonatal pulmonary morbidity | 7.5 | 3.7 | 0.14 |
| -Necrotizing enterocolitis | 0.4 | 0.2 | 0.33 |
| -Seizures | 0.3 | 0.08 | 0.28 |
| -Retinopathy of prematurity | 0.6 | 0.6 | 0.95 |
| -Neonatal infection morbidity | 9.8 | 2.4 | <.001 |
| -Anemia requiring blood transfusion | 1.3 | 0.7 | 0.24 |
| -Neonatal surgery | 0.3 | 0.8 | 0.37 |
| -Hyperbilirubinemia | -0- | 0.03 | --- |
| -Neonatal neurological morbidity | 1.4 | 0.3 | 0.002 |
| -Neonatal death in L&D suite or prior to discharge | 0.4 | 0.3 | 0.63 |
Composite neonatal morbidity includes indented list presented in remaining rows of table.
All results are reported as weighted percentages, p-value from weighted chi-square. The composite neonatal morbidity endpoint was defined by “any yes” or “all no”, as such some item non-response is present for individual components.
Figure 2.
Multivariable modeling to estimate the association between maternal marijuana use and composite neonatal morbidity while adjusting for tobacco use and other sociodemographic factors. Education, participation in Women, Infants and Children Supplemental Nutrition Program, body mass index, and the interaction between tobacco and marijuana were eliminated from the model as non-significant covariates.
Comment
Maternal marijuana use (self-report or presence of THC in the umbilical cord homogenate) was not associated with a composite adverse pregnancy outcome of SGA, SPTB and HTN independent of tobacco use, but was associated with neonatal morbidity.
High quality data regarding the effects of marijuana use in pregnancy are wanting owing to a lack of systematic investigation. The majority of existing studies did not distinguish between medically-indicated and SPTB; however, two recent studies demonstrate an increased risk of SPTB with marijuana use.18, 19 In our study, women who used marijuana had SPTB (preterm labor with or without intact membranes) twice as frequently as those who did not use marijuana (13% vs 6%), although this was not statistically significant. Separation of medically-indicated from SPTB will be important in future studies.
Most prior studies focused on the association between maternal marijuana use and fetal growth. We found no association between marijuana use and SGA. A meta-analysis by English et al published in 1997 found no association between marijuana exposure and low birth weight (pooled odds ratio [OR] 1.09, 95% CI 0.94–1.27).4 Nine of the 10 studies in the meta-analysis used only self-report of marijuana use. Zuckerman et al found an association between THC use and lower birth weight, and suggested that the lack of observed association in other studies may be a result of reliance on self-report.20
In contrast, there are few data regarding the effect of marijuana on hypertensive disorders of pregnancy. We found no increase in hypertensive disorders of pregnancy in women who used marijuana (14 vs 9%). There is limited evidence to evaluate whether marijuana use is associated with pregnancy complications for the mother.8 Future work will need to emphasize timing of exposure and biologic plausibility for outcomes of interest.
A recent systematic review and meta-analysis concluded that marijuana use was not associated with adverse neonatal outcomes defined as birth weight <2500 gms or preterm delivery <37 weeks.7 Secondary outcomes that overlapped with our studied outcomes were level II or greater nursery admission, small for gestational age and perinatal death. Notably, none of the neonatal morbidity outcomes included in our composite were investigated as part of this meta-analysis. The difference between our findings and the published meta-analysis may simply reflect the addition of a number of other neonatal morbidities, including preterm morbidities, in our analysis.
Data regarding preterm morbidity and marijuana use are limited. We found increased neurologic morbidity in neonates exposed to marijuana. Viteri et al found an association between stillbirth and/or cerebral palsy among preterm neonates exposed to illicit drugs,21 while another secondary analysis found no association between marijuana use and neurologic morbidity in preterm neonates.22 Further work is needed to confirm this association, and to determine whether marijuana use causes neonatal morbidity independent of preterm birth.
The observed increase in neonatal infection morbidity has biologic plausibility. There are existing animal data demonstrating changes in the immune response related to cannabis exposure with evidence of immune suppression and T cell dysfunction.23–26 These changes may result from epigenetic modifications, which could result in long-term changes to the immunologic response following in utero exposure.27
While we found an association between neonatal morbidity and marijuana use, we did not find a significant difference in rates of NICU admission (Table 3). This may simply be a reflection of the need for a larger sample size. The higher rates of NICU admission compared to morbidity were predominantly a result of admissions for rule-out infection, or mild respiratory distress including desaturations following delivery. The rates of NICU admission in our study are similar to those published by Warshak et al who identified an increased rate of NICU admission among women (N=6468) who use cannabis compared to non-users (17.2 versus 12.5%).28 A recent systematic review and meta-analysis also noted an increase in NICU admission among neonates exposed to cannabis in utero (pooled OR 2.02, 95% CI 1.27–3.21).6 The observed increased risk of NICU admission in these and other studies29, coupled with our finding of increased neonatal morbidity, emphasizes the need to rigorously evaluate neonatal morbidity in this population.
In our cohort, the agreement between self-reported maternal marijuana use and biological sampling was poor. These findings are consistent with a study from Shiono et al (1995) in which 69% of women with a positive maternal serum screen for THC denied use in a structured interview.30 The unwillingness of pregnant women to disclose use may reflect social desirability bias, or concern for the ramifications of reporting. Regardless of the rationale, marijuana use is under-reported which speaks to the importance of incorporating biologic sampling into future protocols for ascertainment of exposure.
The importance of biological sampling is also emphasized in the National Academies Press publication of the state of the evidence for health effects of cannabis and recommendations for research.8 Umbilical cord homogenate sampling has promise for quantification of use as it performs similarly to meconium testing, and is thought to be a marker of use from the second trimester onward.15 This is in contrast to urine or plasma, which reflect use for days to weeks depending on the chronicity of use. However, there are limited data as to how long THC is detectable in the cord homogenate (thought to be as early as 20 weeks in term infants).13 In addition, despite validation of umbilical cord homogenate testing in other studies, the poor agreement observed may reflect low sensitivity of the assay for THC in women who are less frequent users. Given that the umbilical cord essentially acts as a reservoir for drug metabolites, future work should focus on whether maternal marijuana use can be quantified through sampling of the umbilical cord alone.
Strengths and Limitations
Our study had several limitations. The most important are the infrequency of marijuana use in this cohort, and the lack of available data regarding individual use patterns. The observed lack of an association between marijuana use and the primary composite adverse outcome may be attributable to insufficient sample size. With a fixed sample size of n=48 women who use marijuana and n=1562 non-users, we could detect the observed difference of 10% if the prevalence rates of the adverse outcome were lower (i.e. 15% versus 5% rather than 31% versus 21%) using a two-sided t-test with a significance level of 0.05 and 80% power. However, with the high proportion of adverse outcomes, we did not have sufficient power to detect this difference.
There was poor agreement between self-reported marijuana use and objective documentation, limiting the value of self-reported data. Also, some cases (44%), as part of the parent study design, had no biospecimen data available, potentially introducing bias. Finally, we do not have long-term follow-up data on the neonates to assess for childhood outcomes.
A major strength of the study was the use of biological sampling from umbilical cord homogenate to objectively assess maternal marijuana use. In addition, we had rigorous data collection by trained personnel, population-based sampling resulting in a racially, ethnically, and geographically diverse population, and a large number of cases with adverse pregnancy outcomes.
Conclusions
Further study of the association between maternal marijuana use and adverse perinatal outcomes with biological sampling may help to educate women and providers about the anticipated effects of maternal marijuana use.
Acknowledgments
Financial support: Dr. Metz was supported by the National Institute on Child Health and Human Development under Award Number 2K12HD001271-16. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Presented as a poster presentation at the Society for Maternal-Fetal Medicine 36th Annual Meeting, Las Vegas, NV, January 23-28, 2016.
References
- 1.Volkow ND, Compton WM, Wargo EM. The Risks of Marijuana Use During Pregnancy. JAMA. 2016;317(2):129–30. doi: 10.1001/jama.2016.18612. [DOI] [PubMed] [Google Scholar]
- 2.Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS, et al. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. JAMA. 2016;317(2):207–9. doi: 10.1001/jama.2016.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz TD, Stickrath EH. Marijuana Use in Pregnancy and Lactation: A Review of the Evidence. Am J Obstet Gynecol. 2015;213(6):761–78. doi: 10.1016/j.ajog.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 4.English DR, Hulse GK, Milne E, Holman CD, Bower CI. Maternal cannabis use and birth weight: a meta-analysis. Addiction (Abingdon, England) 1997;92:1553–60. [PubMed] [Google Scholar]
- 5.Jaques SC, Kingsbury A, Henshcke P, et al. Cannabis, the pregnant woman and her child: weeding out the myths. J Perinatol. 2014;34(6):417–24. doi: 10.1038/jp.2013.180. [DOI] [PubMed] [Google Scholar]
- 6.Gunn JKL, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcome: a systematic review and meta-analysis. BMJ Open. 2016;6:e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal Marijuana Use and Adverse Neonatal Outcomes: A Systematic Review and Meta-analysis. Obstet Gynecol. 2016;128:713–23. doi: 10.1097/AOG.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 8.Committee on the Health Effects of Marijuana. An Evidence Review and Research Agenda; Board on Population Health and Public Health Practice; Health and Medicine Division; National Academies of Sciences. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. http:www.nap.edu/246252017.9. [PubMed]
- 9.Varner MW, Silver RM, Rowland Hogue CJ, et al. Association between stillbirth and illicit drug use and smoking during pregnancy. Obstet Gynecol. 2014;123:113–25. doi: 10.1097/AOG.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker CB, Hogue CJ, Koch MA, et al. Stillbirth Collaborative Research Network: design, methods and recruitment experience. Paediatr Perinat Epidemiol. 2011;25:425–35. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexander GR, Himer JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 12.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed May 5, 2017];United States Drug Testing Laboratories, Inc. http://www.usdtl.com/testing/umbilical-cord-tissue-drug-test-labs.
- 14.Moore C, Rana S, Coulter C, Feyerherm F, Prest H. Application of two-dimensional gas chromotography with electron capture chemical ionization mass spectrometry to the detection of 11-nor-delta-9-tetrahydrocannabinol-9-carboxylic acid (THC-COOH) in hair. Journal of Analytical Toxicology. 2006;30:171–7. doi: 10.1093/jat/30.3.171. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. 2006;26:11–4. doi: 10.1038/sj.jp.7211416. 16. [DOI] [PubMed] [Google Scholar]
- 16.Pinar H, Koch MA, Hawkins H, et al. The Stillbirth Collaborative Research Network (SCRN) placental and umbilical cord examination protocol. Am J Perinatol. 2011;28:781–92. doi: 10.1055/s-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller EI, Norris HRK, Rollins DE, Tiffany ST, Wilkins DG. A novel validated procedure for the determination of nicotine, eight nicotine metabolites and two minor tobacco alkaloids in human plasma or urine by solid-phase extraction coupled with liquid chromotography-electrospray ionization-tandem mass spectrometry. J Chromatogr. 2010;878:725–37. doi: 10.1016/j.jchromb.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saurel-Cubizolles MJ, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG. 2014;121:971–7. doi: 10.1111/1471-0528.12626. [DOI] [PubMed] [Google Scholar]
- 19.Dekker GA, Lee SY, North RA, McCowan LM, Simpson NA, Roberts CT. Risk factors for preterm birth in an international prospective cohort of nulliparous women. PloS one. 2012;7:e39154. doi: 10.1371/journal.pone.0039154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuckerman B, Frank DA, Hingson R, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320:762–8. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]
- 21.Viteri OA, Mendez-Figueroa H, Pedroza C, Leon MG, Sibai BM, Chauhan SP. Relationship between self-reported maternal substance abuse and adverse outcomes in the premature newborn. Am J Perinatol. 2016;33(2):165–71. doi: 10.1055/s-0035-1563549. [DOI] [PubMed] [Google Scholar]
- 22.Dotters-Katz SK, Smid MC, Manuck TA, Metz TD. Risk of neonatal and childhood morbidity among preterm infants exposed to marijuana. J Matern Fetal Neonatal Med. 2017 doi: 10.1080/14767058.2016.1269165. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J. Neuroimmunol. 1998;83:116–123. doi: 10.1016/s0165-5728(97)00227-0. 1998. [DOI] [PubMed] [Google Scholar]
- 24.Cabral GA, Vasquez R. Effects of marijuana on macrophage function. Adv. Exp. Med. Biol. 1991;288:93–105. doi: 10.1007/978-1-4684-5925-8_10. [DOI] [PubMed] [Google Scholar]
- 25.Klein TW, Newton C, Friedman H. Resistance to Legionella pneumophila suppressed by the marijuana component, tetrahydrocannabinol. J. Infect. Dis. 1994;169:1177–1179. doi: 10.1093/infdis/169.5.1177-a. 1994. [DOI] [PubMed] [Google Scholar]
- 26.Cabral GA, Marciano-Cabral F. Cannabinoid-mediated exacerbation of brain infection by opportunistic amebae. J Neuroimmunol. 2004;147(1–2):127–30. doi: 10.1016/j.jneuroim.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Zumbrun EE, Sido JM, Nagarkatti PS, Nagarkatti M. Epigenetic regulation of immunological alterations following prenatal exposure to marijuana cannabinoids and its long term consequences in offspring. J Neuroimmune Pharmacol. 2015;10(2):245–54. doi: 10.1007/s11481-015-9586-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warshak CR, Regan J, Moore B, Magner K, Kritzer S, Van Hook J. Association between marijuana use and adverse obstetrical and neonatal outcomes. J Perinatol. 2015;35:991–5. doi: 10.1038/jp.2015.120. [DOI] [PubMed] [Google Scholar]
- 29.Hayatbakkhsh MR, Flenady VJ, Gibbons KS, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71(2):215–9. doi: 10.1038/pr.2011.25. [DOI] [PubMed] [Google Scholar]
- 30.Shiono PH, Klebanoff MA, Nugent RP, et al. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. Am J Obstet Gynecol. 1995;172(1 Pt 1):19–27. doi: 10.1016/0002-9378(95)90078-0. [DOI] [PubMed] [Google Scholar]