Figure 4. KPC-1 and CHIN-1 act in NR pioneers at the onset of NR assembly.
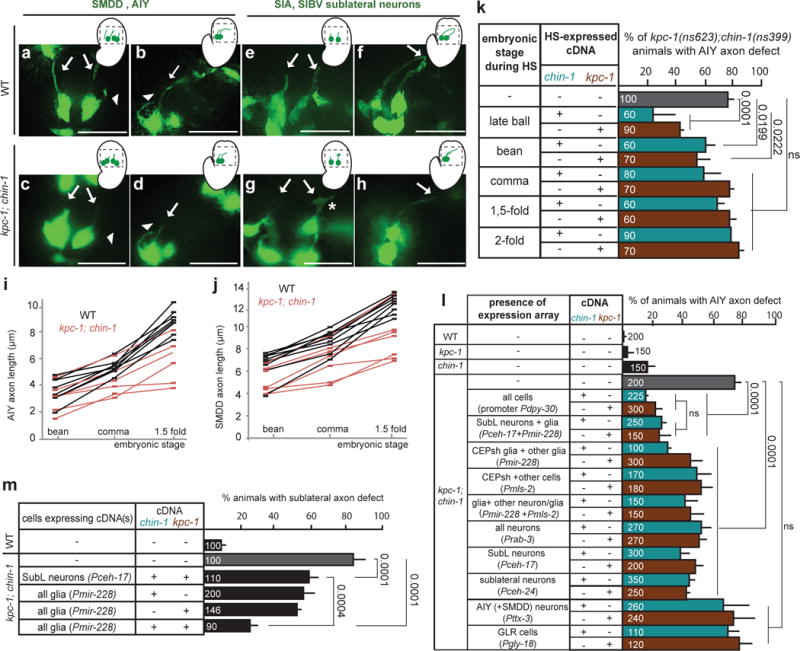
(a–j) Extension of AIY axons and SubL commissure axons into the presumptive nerve-ring is delayed in kpc-1; chin-1 mutants compared to wild-type embryos. Histograms and scale bars, as in Fig. 3. (a–h) Head region (outlined in schematics) of bean or 1.5 fold embryos expressing Pttx-3::GFP (a-d) or Pceh-17::GFP (e-h). Arrows: SubL axons, arrowhead: AIY axon. Asterisk: growth cone. (i–j) Squares: individual axon measurement at given embryonic stage. Lines track individuals across stages. Number of animals analyzed: (i) n=7 for WT, n=6 for chin-1; kpc-1 mutants, (j) n=8 for WT, n=7 for chin-1; kpc-1 mutants. (k) CHIN-1 and KPC-1 expression is necessary prior to the embryonic comma stage for proper nerve-ring assembly. HS: Heat-Shock driving chin-1 or kpc-1 cDNA expression. (l,m) CHIN-1 and KPC-1, acting non-cell-autonomously from SubL neurons and glia, can rescue chin-1; kpc-1 mutant defects of follower axons. Furthermore, CHIN-1 and KPC-1 acting non-cell-autonomously from glia can rescue chin-1; kpc-1 mutant defects of pioneer SubL axons. Rescue of mutant defects by cDNA expression using indicated promoters (Expression patterns are described in Supplementary Methods and Supplementary Tables S8, S10). The following alleles are used unless otherwise indicated: kpc-1(gk8), chin-1(ns399). (k–m) Numbers inside bars: total animals scored per genotype, n=3 independent scoring experiments or number of transgenic lines in rescue experiments. Mean +/− Error bars: SEM. Numbers above bars of significance, p values from Fisher’s exact test. ns: non significant.
