Fig.6. cPT binding and thermodynamic profile of monomeric wild type gp120 YU-2 for cPTs 3 (A and C) and 36 (B and C).
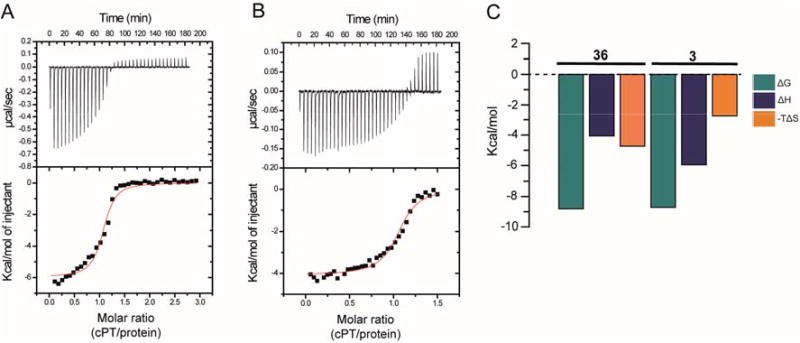
Compound solution, 390 μM (for 3) or 200 μM (for 36), was titrated in 8 μL steps into 30 μM of YU2 full-length gp120 at 25°C in the reaction chamber of a VP-ITC instrument using 1× PBS buffer at pH 7.3. The resulting heat changes were integrated, and the values obtained were fitted to a quadratic binding equation (one site binding model). The following Kd and thermodynamic values were derived: 3 (A and C): KD = 415 ± 98 nM, n = 1.06 ± 0.02, ΔH = −5.96 ± 0.13 kcal/mol, ΔS = 9.22 cal/mol; 36 (B and C): KD = 350 ± 62 nM, n = 1.06 ± 0.01, ΔH = −4.07 ± 0.06 kcal/mol, ΔS = 15.9 cal/mol.
