Table 1. Structure activity relationship around the triazole moiety.
A general structure is shown with the R group specified in the table, each with the HIV-1Bal.01 infection inhibition IC50 value. Asterisk (*) indicates that the cPT was made “in house” using a synthetic alkyne that was incorporated in the click reaction during the cPT synthesis.
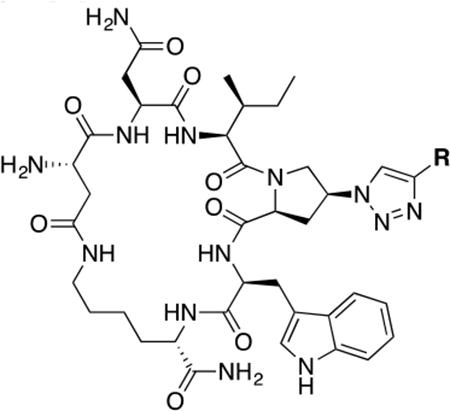
| |||
|---|---|---|---|
| Compound/R group | HIV-1 IC50 (nM) | Compound/R group | HIV-1 IC50 (nM) |
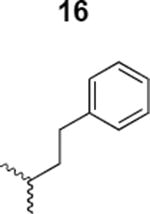
|
2018 ± 9520 |
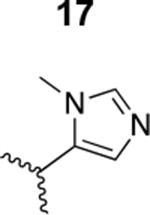
|
Inactive |
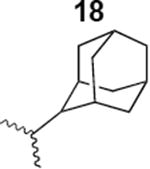
|
1400 ± 200 |
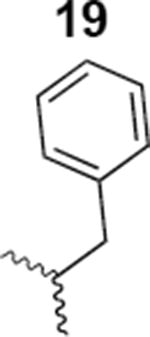
|
438 ± 12 |
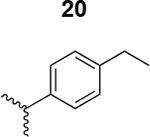
|
1200 ± 500 |
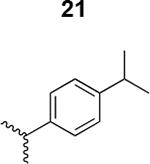
|
900 ± 45 |
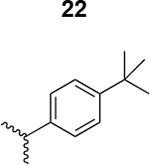
|
350 ± 25 |
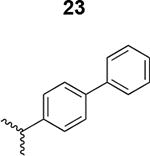
|
269 ± 32 |
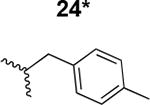
|
180 ± 9 |
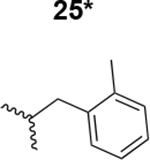
|
5100 ± 220 |
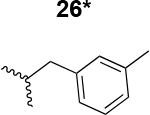
|
220 ± 42 |
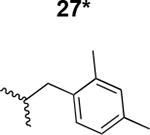
|
302 ± 50 |
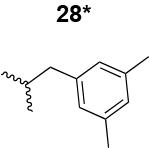
|
2000 ± 390 |
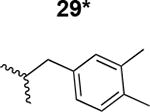
|
8000 ± 250 |
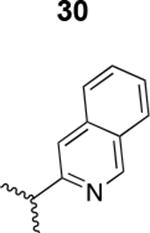
|
5000 ± 850 |
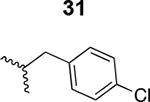
|
1800 ± 500 |
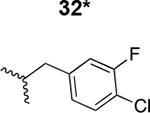
|
7300 ± 740 |
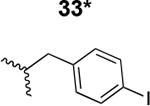
|
6000 ± 450 |
