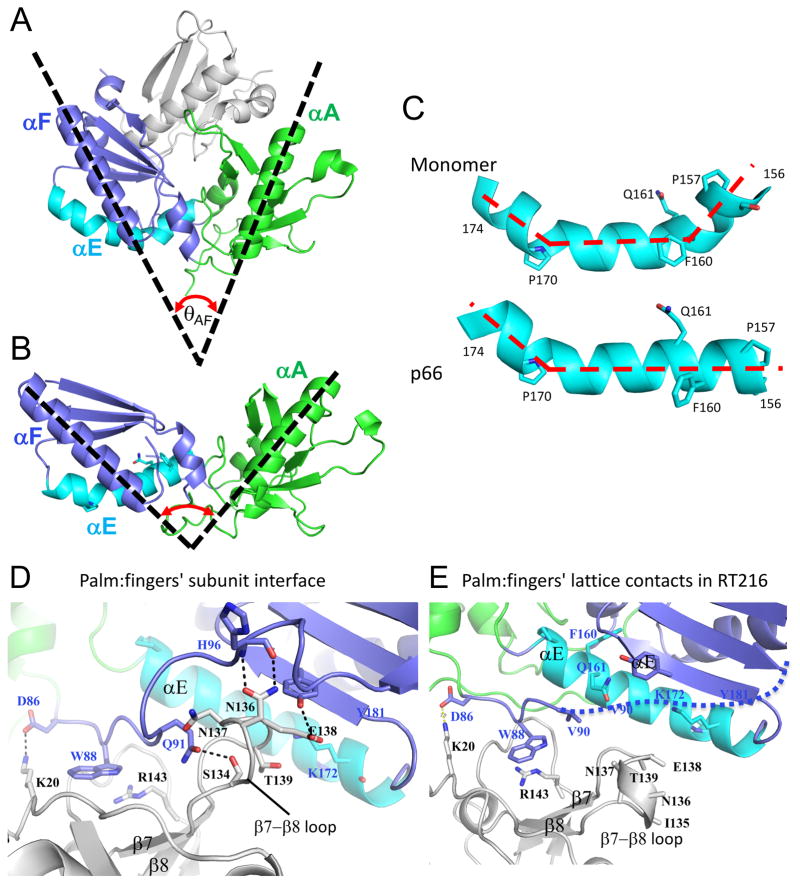
Figure 2. Structural comparisons of the palm/loop domains.
A) Structure of the complex formed by the fingers (green)/palm (blue) and connection (gray) domains in the p51ΔPL monomer (pdb: 4KSE), and helix αE is indicated in cyan. The angle θAF formed by helices A and F is ~ 50°. B) Ribbon diagram of the isolated RT216 (pdb: 1HAR, [42] construct that includes the fingers and most of the palm domains color coded as in A. For the isolated construct, θAF expands to ~100°. C) Structural comparison of palm domain helix E (residues 156-174) in the structures shown in A and B, illustrating elimination of the bend near Phe160, Gln161. D) Ribbon diagram showing the region of the p66/p51 subunit interface formed from the p51 fingers (gray) and the p66 palm (blue) domains (pdb: 1DLO). The p66 fingers domain (green) does not contribute to the interface. E) Ribbon diagram showing the lattice contacts in the RT216 crystal structure that correspond to the region shown in panel D. The region of the p66/p51 interface formed near palm residue W88 also forms a lattice contact, however the region of the interface involving β7–β8 does not.