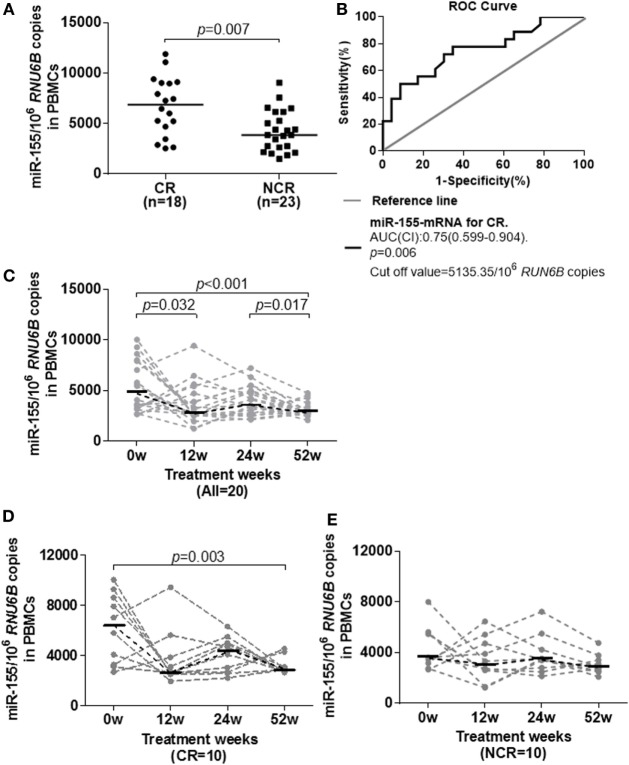
Figure 4.
Longitudinal analysis of miR-155 expression in peripheral blood mononuclear cells (PBMCs) of IA patients during telbivudine therapy. (A) miR-155 mRNA levels in PBMCs at baseline in the CR and NCR groups. (B) Receiver-operating characteristic (ROC) curve showed the suitability of miR-155 mRNA levels at baseline to predict a CR to telbivudine therapy. An area under the curve (AUC) of 1.0 occurs with an ideal test, whereas an AUC of <0.5 indicates a test of no diagnostic value. (C) Temporal dynamics of miR-155 mRNA levels in PBMCs of all patients. (D) Comparison of miR-155 mRNA levels among individuals in the CR group. (E) Comparison of miR-155 mRNA levels among individuals in the NCR group. IA, immune activation; CR, complete response; NCR, non-complete response.