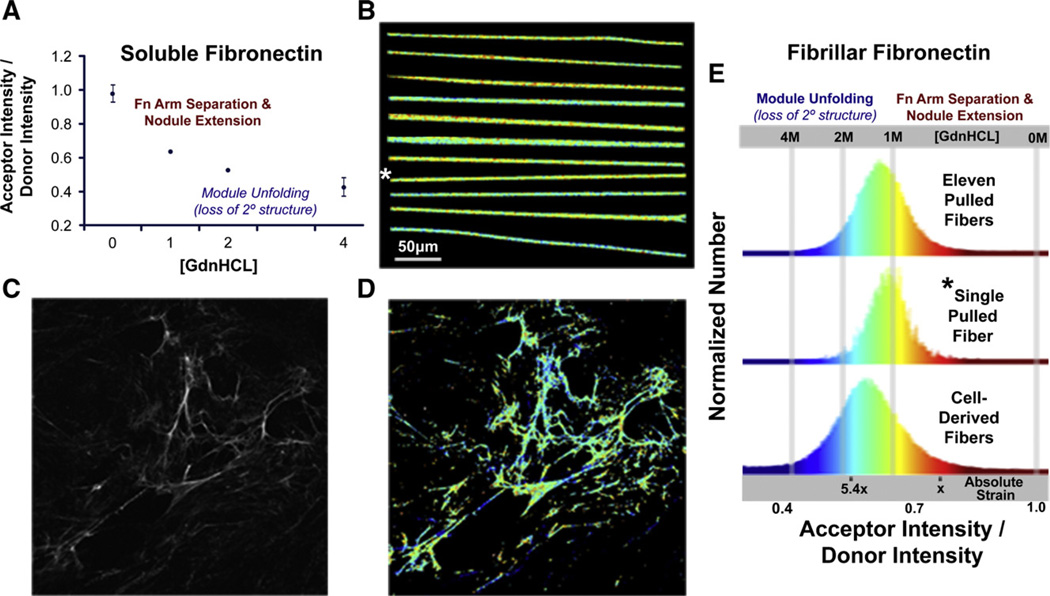
Fig. 2.
Spatially resolved conformational mapping by Fluorescence Resonance Energy Transfer (FRET) and comparison of FRET histograms of manually deposited fibronectin and extracellular matrix fibers of living fibroblasts. (A) Plasma fibronectin, dual-labeled with donors and acceptors (Fn-d/a—see Materials and methods), is subjected to increasing concentrations of guanidine hydrochloride (GdnHCL) to calibrate a ratio of the acceptor intensity divided by the donor intensity (IA/ID) to known conformational states of fibronectin in solution. In concentrations greater than 1.5 M GdnHCL, circular dicroism indicates that fibronectin undergoes a rapid and significant loss of secondary structure causing the Fn modules to unfold (Alexander et al., 1979; Baugh and Vogel, 2004). (B) Reproducibility of conformational distribution: 11 manually deposited Fn fibers containing 5% Fn-d/a were laid onto a transparent sheet of silicone and the pixel-resolved IA/ID values were mapped to a color gradient representing module-unfolded (blue) or dimer-arm separated (red) Fn conformations. Images of individually deposited fibers here were mounted next to each other for presentation purposes (though it is possible manually, see Fig. 5). (C) A fluorescence image of a 24-h cell-derived Fn matrix derived from NIH-3T3 fibroblasts (Baneyx et al., 2001) is shown beside the (D) corresponding false color FRET map. (E) The top histogram represents IA/ID data collected from every pixel analyzed from the eleven fibers shown in (B). The middle histogram shows the IA/ID data collected from the single fiber in (B) as denoted by the *. The bottom histogram shows the IA/ID data collected from the cell-derived Fn matrix image in (D). The absolute strain scale represents the average IA/ID values of Fn fibers, from fully relaxed (nodules of quatenary structure are present), to extended, to unfolded, to the point where they begin to break (5.4×), as described in the text and in Fig. 3. Scale bar in (B) is the same scale as the images in (C) and (D).