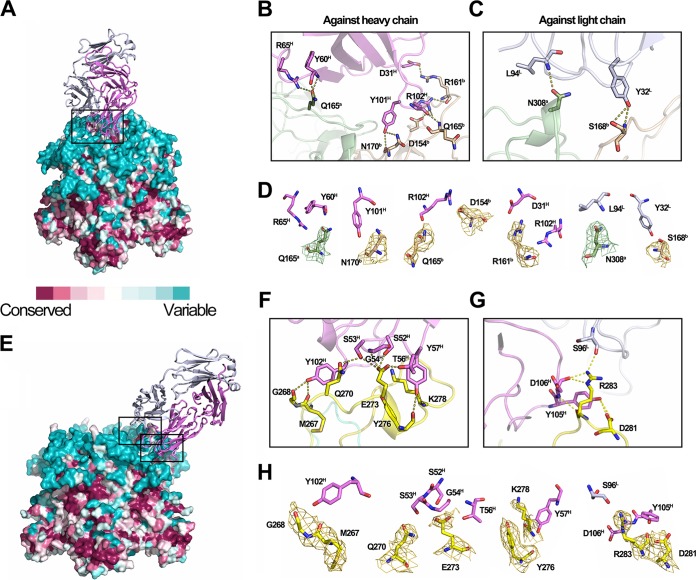
FIG 3 .
Antigen-antibody interactions in the HPV58p:A12A3 and HPV59p:28F10 complexes. (A and E) Surface representations of HPV58 and -59 pentamers (HPV58p and -59p) colored as per the sequence conservation, which is based on alignment of multiple HPV L1 sequences (see Fig. S8 in the supplemental material). In panel E, four of the binding 28F10 Fabs are omitted for clarity. Close-up views of the interfaces are shown in panels B and C for HPV58p:A12A3 and panels F and G for HPV59p:28F10. (B and C) The interactions of HPV58 against the heavy chain (B) and light chain (C) of MAb A12A3 are shown. (F and G) Magnified views of the upper (F) and lower (G) boxed regions in panel E. Side chains involved in the interaction between antigen and antibody are labeled and shown as sticks. (D and H) All contacts are depicted as dotted lines. Individual contacts in the interface of HPV58p:A12A3 (D) and HPV59p:28F10 (H) are displayed with the sample electron density (2Fo-Fc) maps contoured at 1σ above the mean shown for the epitope residues of HPV.