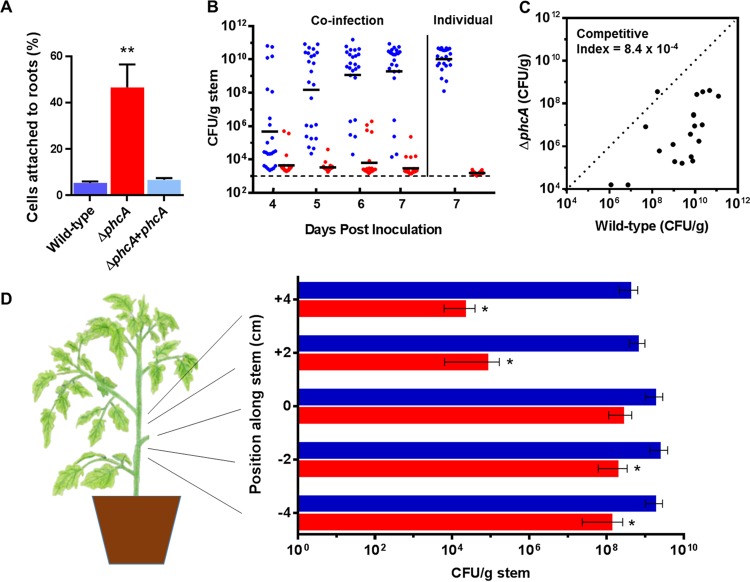
FIG 7 .
The low-cell-density-mode-mimicking R. solanacearum ΔphcA mutant hyperattached to tomato roots but had reduced competitive fitness in planta and was defective in plant colonization. (A) Root attachment. Four-day-old tomato seedlings were incubated for 2 h in water with wild-type, ΔphcA mutant, or complemented ΔphcA mutant cells and were then rinsed, ground, and dilution plated to quantify attached bacteria. Results reflect three biological replicates, each performed with 10 technical replicates (n = 30). The asterisk indicates that the ΔphcA mutant strain attached better to roots than the wild-type and complemented mutant strains (P < 0.0001, ANOVA). (B) Competitive fitness of wild-type versus ΔphcA bacteria following soil-soak inoculation. Unwounded tomato plants were coinoculated by soaking soil with 1:1 suspensions of reciprocally marked wild-type and ΔphcA bacteria. Tomato mid-stems were sampled over time, and the population size of each strain was determined by serial dilution plating on relevant selective media. For comparison, bacterial population sizes in plants inoculated with only one strain are shown on the right. The wild-type strain outcompeted the ΔphcA mutant at all time points (P < 0.0001 [Wilcoxon signed-rank test; n = 12 plants per inoculation per time point]). (C) Competitive fitness of ΔphcA and wild-type bacteria in tomato stems following direct stem inoculation. Tomato plants were coinoculated via a cut leaf petiole with 2,000 CFU of reciprocally marked wild-type and ΔphcA bacteria in a 1:1 suspension. At 72 hpi, the population size of each strain 4 cm above the inoculation site was determined by dilution plating. The median competitive index value determined for the ΔphcA mutant was 8.4× 10−4 (P < 0.0001 [Wilcoxon signed-rank test; n = 10 plants per coinoculation, 20 total]). (D) Dispersal of wild-type and ΔphcA strains in tomato stems. Petioles of 3-week-old tomato plants were inoculated with 2,000 cells of either wild-type or ΔphcA bacteria. After 72 h, stem sections were harvested at 0, 2, and 4 cm above and below the point of inoculation, and bacterial population sizes were determined by dilution plating. Asterisks indicate P < 0.01 (Wilcoxon signed-rank test; n = 8 plants per treatment). Bars represent standard errors of the means.