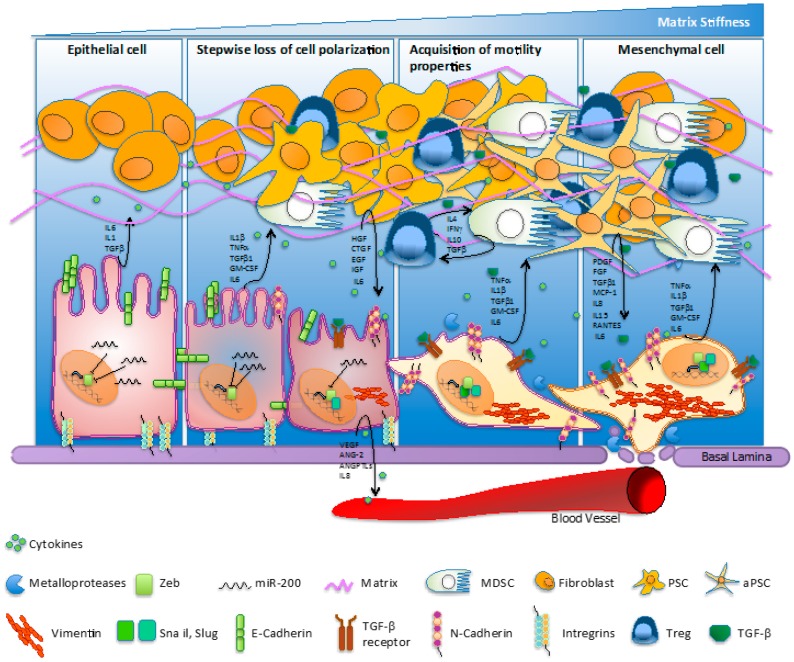
Figure 1.
Molecular hallmarks/fluctuations/switching regulating the epithelial-to-mesenchymal transition (EMT) process in pancreatic cancer. The EMT process involves loss of cell polarization, a gain in migratory abilities and progressive acquisition of a mesenchymal phenotype. The EMT mechanism is characterized by the ‘cadherin switch’, where E-cadherin expression is progressively downregulated and replaced by the expression of N-cadherin. The transition process is associated to a decrease of miR-200 levels and an increase of classical E-cadherin transcriptional suppressors—such as ZEB1, Snail, and Slug—activated upstream by TGF-β. Cells undergoing EMT commonly quit the expression of extracellular matrix (ECM) elements mediating structural rigidity and cell adhesion in favor of proteases, cytokines, growth factors, and ECM components which improve cell migration and intravasation in bloodstream. Pancreatic cancer cell cytokines accelerate transformation of fibroblasts into quiescent pancreatic stellate cells (PSCs) and then into activated pancreatic stellate cells (aPSCs). Furthermore, inflammatory cytokines recruit myeloid progenitor cells and mediate their subsequent differentiation into myeloid-derived suppressive cells (MDSCs), which suppress the immune surveillance function. IL: interleukin; TGF: transforming growth factor; TNF: tumor necrosis factor; GM-CSF: granulocyte-macrophage colony-stimulating factor; HGF: hepatocyte growth factor; CTGF: connective tissue growth factor; EGF: epidermal growth factor; IFN: interferon; PDGF: platelet-derived growth factor; MCP-1: macrophage inflammatory protein 1; RANTES: regulated upon activation normally T-expressed and presumably secreted; VEGF: vascular endothelial growth factor; ANG-2: angiopoietin-2; ANGPTL: angiopoietin-like.