Abstract
Objectives
Deficient plasminogen activator inhibitor-1 (PAI-1) prevented hypertension in mice. Plasma PAI-1 was associated with hypertension in cross-sectional analyses, but the prospective association of PAI-1 with incident hypertension in large epidemiological studies is scarce.
Methods
Leveraging two longitudinal cohorts of American Indians in the Strong Heart Study (SHS, N = 1019) and the Strong Heart Family Study (SHFS, N = 1502), we examined the prospective association of plasma PAI-1 with incident hypertension by multivariate logistic regression, adjusting for age, sex, study site, smoking, drinking, dietary sodium, obesity, lipids, fasting glucose, kidney function, inflammation, and follow-up years. Family relatedness in the SHFS was accounted for using the GLIMMIX procedure. Plasma PAI-1 level at baseline was measured by immunoassay. All participants were free of hypertension, cardiovascular diseases, and chronic kidney disease at baseline.
Results
A total of 305 and 258 participants, respectively, from the SHS (57 ± 7 years) and the SHFS (33 ± 13 years) developed incident hypertension during follow-up. In the SHS, higher level of log-transformed PAI-1 was associated with 1.35-fold increased risk of hypertension [odds ratio (OR) (95% confidence interval): 1.35 (1.06–1.72)]. Analysis using categorical PAI-1 (in tertiles) showed that participants in the highest tertile (≥ 58 ng/ml) had 63% increased risk for hypertension [OR = 1.63 (1.12–2.37)] compared with those in the lowest tertile (<33 ng/ml). This association was confirmed in the SHFS with similar effect sizes [OR = 1.41 (1.11–1.81) for log-transformed PAI-1; OR = 1.64 (1.08–2.50) for categorical PAI-1: ≥ 58 vs. <33 ng/ml].
Conclusion
A higher level of plasma PAI-1 is significantly associated with hypertension in American Indians, independent of established risk factors. The potential causality warrants further investigation.
Keywords: American Indians, hypertension, plasminogen activator inhibitor-1, prospective association, the Strong Heart Study
INTRODUCTION
Plasminogen activator inhibitor-1 (PAI-1) is a serine protease inhibitor mainly produced by endothelial cells, vascular smooth muscle cells, and hepatocytes [1]. In vascular tissue, PAI-1 promotes accumulation of extracellular matrix and regulates vascular remodeling and perivascular fibrosis [2]. In plasma, PAI-1 promotes clot formation, thereby contributing to myocardial infarction, stroke, and other cardiovascular diseases (CVD) [3]. Accumulating evidence suggests that PAI-1 may be implicated in the pathogenesis of hypertension [4]. For instance, recent experimental studies have demonstrated that PAI-1 deficiency prevented hypertension and vascular fibrosis in mice [2,5–7]. Associations of plasma PAI-1 with hypertension [8–14] and its related conditions, such as arterial stiffness [15] and atherosclerosis [16], have been reported in human studies. However, these results were largely derived from cross-sectional analyses and thus raising a question as to whether increased plasma PAI-1 is a cause or consequence or just an accompanied manifestation of hypertension. To date, we are aware of only one study investigating the prospective association of plasma PAI-1 with incident hypertension in the Framingham Offspring Study [17]. The prognostic effect of plasma PAI-1 on hypertension risk has not been well studied, and no study has investigated the possible role of PAI-1 in hypertension among American Indians, a minority group with high rates of CVD, diabetes, hypertension, and related conditions [18]. The goal of this study is to examine the prospective association of plasma PAI-1 with risk of hypertension in two longitudinal cohorts of American Indians participating in the Strong Heart Study (SHS) and the Strong Heart Family Study (SHFS).
MATERIALS AND METHODS
Study population
The SHS is a multicenter, population-based longitudinal study of CVD, diabetes, and associated risk factors in American Indians in tribes and communities residing in Arizona, North/South Dakota, and Oklahoma. The study design, survey methods, and laboratory techniques of SHS have been described previously [19]. Briefly, the SHS first clinical examination was conducted in 1989–1991 (Phase I) by enrolling 4549 tribal members aged 45–74 years. All surviving participants were re-examined in 1993–1995 (Phase II) and 1998–1999 (Phase III). The SHFS, a component of SHS, enrolled 3665 participants from 94 families of the original SHS cohort members in 2001–2003 (Phase IV). All living SHFS participants were re-examined in 2006–2009 (Phase V) and are currently being followed through 2018. The SHS and SHFS protocols were approved by the Institutional Reviews Boards from the Indian Health Service and the participating institutions.
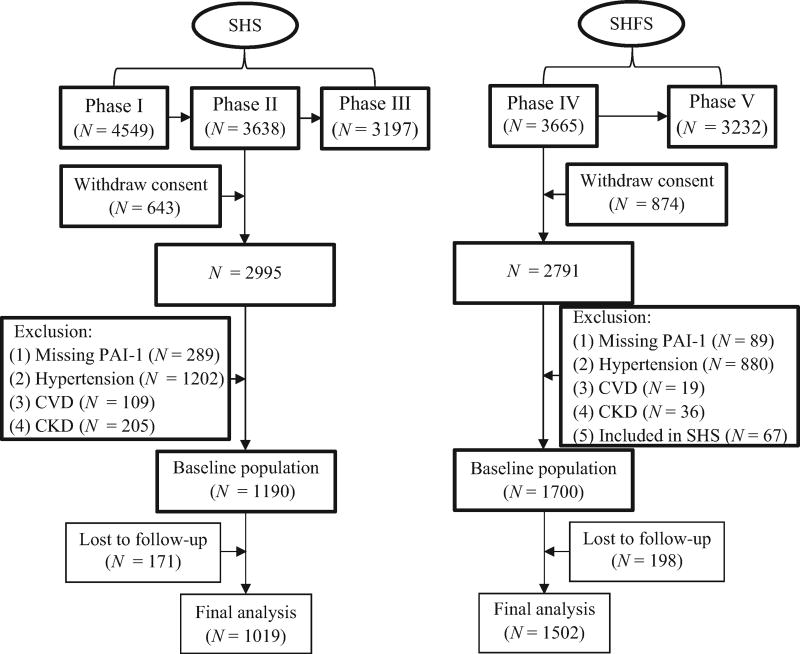
Figure 1 described the selection of study participants included in the current analysis. In the SHS, given that plasma PAI-1 was only available in Phase II, we studied 3638 participants who attended this clinical visit. Of these, 643 participants from one community were excluded due to withdraw of consent. Participants were further excluded if they met one or more of the following: missing plasma PAI-1 (N = 289); prevalent hypertension (N = 1202); overt CVD (N = 109); chronic kidney disease (N = 205); or loss of follow-up (N = 171). Our final data analysis in the SHS included a total of 1019 participants. In the SHFS, we excluded participants who withdrew their consent (N = 874), had no plasma PAI-1 data (N = 89), had prevalent hypertension (N = 880), CVD (N = 19) or chronic kidney disease (N = 36), also attended the SHS (N = 67), or lost to follow-up (N = 198). A total of 1502 participants were included in the final data analysis of the SHFS. We excluded participants with prevalent CVD or chronic kidney disease because they might have received medications that could affect blood pressure (BP) or plasma PAI-1 levels [20].
FIGURE 1.
Flowchart illustrating the selection of study participants. CKD, chronic kidney disease; CVD, cardiovascular disease; PAI-1, plasminogen activator inhibitor-1; SHFS, the Strong Heart Family Study; SHS, the Strong Heart Study.
Measurement of plasma plasminogen activator inhibitor-1
Plasma PAI-1 concentrations were measured using ELISA according to standard protocols as described previously [21]. All antibodies and reagents were obtained from the Center for Thrombosis and Vascular Research, University of Leuven, Belgium. The assay coefficient of variation was less than 5%.
Definition of incident hypertension
BP was measured three times by trained staff using a standard mercury sphygmomanometer and a cuff of appropriate size according to a standard protocol [22], after the participants had been resting for at least 5 min. The first and fifth Korotkoff sounds were recorded as SBP and DBP. The mean of the last two measurements was used in statistical analyses. Hypertension was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg or under antihypertensive medications [22]. Incident hypertension was defined as participants who were free of hypertension at baseline but initiated antihypertensive medications during follow-up or had an SBP at least 140 mmHg or an DBP at least 90 mmHg in the last follow-up examination. Incident cases of hypertension were identified through follow-up of participants in examinations conducted in 1998–1999 (SHS, Phase III) or 2006–2009 (SHFS, Phase V) and verified by review of medical records as previously described [23].
Assessment of hypertension risk factors
Cigarette smoking was classified as current smoking, past smoking, and never smoking. Current smoking was defined as having smoked at least 100 cigarettes in the entire life, having smoked cigarettes regularly, and smoking currently. Past smoking was defined as having smoked at least 100 cigarettes in the entire life, having smoked cigarettes regularly in the past, but not smoking currently. Never smoking was defined as never smoked or having smoked fewer than 100 cigarettes in the entire life. Alcohol consumption was classified as current drinkers, former drinkers, and never drinkers as previously described [24]. Current drinkers were those who had consumed any alcohol during the past year, former drinkers had stopped consuming alcohol for at least 12 months, and never drinkers were those who reported never drinking alcohol in their life time. Dietary intake was assessed using a 24-h dietary recall in the SHS and a Block food frequency questionnaire and an American Indian supplemental foods questionnaire that collected data regarding reported ‘usual’ intake of 119 foods over the past year in SHFS [25]. Dietary sodium (mg/day) was extracted from the dietary records. Body weight (kg) and height (cm) were measured when participants wore light clothes and no shoes by trained staff. BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). Obesity was defined as a BMI at least 30 kg/m2, and overweight was defined as a BMI over 25 kg/m2 but less than 30 kg/m2 [26]. Fasting glucose, serum creatinine, C-reactive protein, and blood lipids, including total cholesterol, triglycerides, LDL cholesterol (LDL-C), and HDL cholesterol (HDL-C), were measured by standard laboratory methods [19]. Estimated glomerular filtration rate (eGFR) was estimated on the basis of serum creatinine by using the Modification of Diet in Renal Disease Study equation [27]. Chronic kidney disease was defined as an eGFR less than 60 ml/min per 1.73m2.
Statistical analysis
Baseline characteristics were presented according to plasma PAI-1 levels in the SHS participants and the SHFS participants, respectively. PAI-1 levels were categorized into three levels in both cohorts – low: less than 33 ng/ml, intermediate: 33–57 ng/ml, and high: at least 58 ng/ml (in tertiles in the SHS participants). Log-transformation was applied to maximal normality of plasma PAI-1. A two-tailed P value less than 0.05 was considered statistically significant. All statistical analysis was conducted using SAS statistical software (version 9.4; Cary, North Carolina, USA).
Analysis in the Strong Heart Study
To examine the prospective association between baseline plasma PAI-1 and incident hypertension, we constructed a multivariate logistic regression model, in which incident hypertension was the dependent variable and baseline plasma PAI-1 (continuous log-transformed PAI-1 or categorical PAI-1 levels in tertiles: low vs. intermediate vs. high) was the independent variable, adjusting for conventional risk factors, including age, sex, study site, current smoking, current drinking, dietary sodium, obesity, fasting glucose, LDL-C, HDL-C, eGFR, C-reactive protein at baseline as well as follow-up years. Missing data were imputed by multiple imputations using the Markov Chain Monte Carlo (MCMC) method.
Replication in the Strong Heart Family Study
To confirm the prognostic role of plasma PAI-1 in predicting incident hypertension identified in the SHS, we constructed a similar multivariate logistic regression model in the SHFS. In this model, incident hypertension was the dependent variable and baseline plasma PAI-1 (continuous log-transformed PAI-1 or categorical PAI-1 levels as defined above: low vs. intermediate vs. high) was the independent variable, adjusting for age, sex, study site, current smoking, current drinking, dietary sodium, obesity, fasting glucose, LDL-C, HDL-C, eGFR, C-reactive protein, and follow-up years. Multiple imputations were conducted to impute missing data using the MCMC method. Family relatedness of study participants was accounted for using the GLIMMIX procedure implemented in SAS.
Sensitivity analyses
To examine whether potential misclassification of incident hypertension influences our results, we considered more restrictive definitions of incident hypertension that included participants who initiated antihypertensive medications during follow-up or had an SBP at least 160 mmHg or an DBP at least 95 mmHg by the end of follow-up. To examine whether baseline BP affects the relationship between plasma PAI-1 and risk of hypertension, we additionally adjusted for baseline SBP. To examine whether diabetes affects our results, except for adjusting for fasting glucose in the above-described models, we also conducted separate analysis by excluding participants with prevalent diabetes at baseline. To examine whether plasma PAI-1 improves prediction performance over conventional risk factors, such as age, sex, smoking, drinking, dietary sodium, obesity, fasting glucose, LDL-C, HDL-C, eGFR, and C-reactive protein, we calculated and compared area under the receiver operating characteristic curves for two prediction models (conventional risk factors only vs. conventional risk factors plus log-transformed PAI-1) using logistic regression.
RESULTS
Baseline characteristics
Our analyses included 1019 SHS participants (mean age 57 ± 7 years, 61% women) and 1502 SHFS participants (mean age 33 ± 13 years, 62% women). The median levels of plasma PAI-1 in the two cohorts were similar [44 ng/ml [interquartile range (IQR): 29–68] in the SHS, 44 ng/ml (IQR: 26–69) in the SHFS]. Baseline characteristics of study participants in the two cohorts are shown in Tables 1 and 2, respectively. Participants with higher plasma PAI-1 had significantly higher BP at baseline than those with lower plasma PAI-1 in both cohorts (all P < 0.05). Compared with participants with lower level of plasma PAI-1, those with higher plasma PAI-1 were older and had higher levels of BMI, fasting glucose, blood lipids, eGFR, and C-reactive protein in both cohorts (all P < 0.05). No significant difference in dietary sodium or cigarette smoking was found across PAI-1 levels in both studies.
TABLE 1.
Baseline characteristics of the Strong Heart Study participants according to plasma plasminogen activator inhibitor-1 levels (1993–1995, N = 1019)
| Characteristics | Level of plasma PAI-1 (ng/ml) | P value for linear trend | ||
|---|---|---|---|---|
| Low (4–32) | Intermediate (33–57) | High (58–479) | ||
| N | 320 | 349 | 350 | |
| Age (years) | 56.6 ± 6.4 | 57.6 ± 7.0 | 58.6 ± 7.6 | <0.001 |
| Male [n (%)] | 147 (45.94) | 133 (38.11) | 117 (33.43) | 0.001 |
| Current smoking [n (%)] | 131 (40.94) | 137 (39.26) | 153 (43.71) | 0.451 |
| Current drinking [n (%)] | 116 (36.25) | 112 (32.09) | 129 (36.86) | 0.840 |
| Obesity [n (%)] | 100 (31.25) | 184 (52.72) | 217 (62) | <0.001 |
| Dietary sodium (mg/day) | 3084.5 ± 1635.2 | 3114.2 ± 1637.2 | 3222.3 ± 1709.4 | 0.293 |
| SBP (mmHg) | 115.7 ± 12.5 | 118.7 ± 11.4 | 119.1 ± 11.2 | <0.001 |
| DBP (mmHg) | 70.3 ± 8.3 | 72.3 ± 7.5 | 71.8 ± 8.0 | 0.012 |
| BMI (kg/m2) | 28.1 ± 5.4 | 31.0 ± 5.9 | 31.7 ± 5.6 | <0.001 |
| Fasting plasma glucose (mg/dl) | 122.3 ± 59.6 | 141.1 ± 72.0 | 149.2 ± 75.8 | <0.001 |
| Total cholesterol (mg/dl) | 193.6 ± 38.6 | 194.3 ± 37.2 | 194.9 ± 37.0 | 0.657 |
| Triglycerides (mg/dl) | 125.5 ± 81.5 | 152.8 ± 107.5 | 176.6 ± 131.5 | <0.001 |
| LDL-cholesterol (mg/dl) | 123.7 ± 34.2 | 124.9 ± 33.0 | 121.3 ± 31.2 | 0.362 |
| HDL-cholesterol (mg/dl) | 45.2 ± 14.7 | 40.5 ± 13.0 | 39.7 ± 12.4 | <0.001 |
| eGFR (ml/min per 1.73m2) | 83.0 ± 21.2 | 85.4 ± 28.2 | 87.6 ± 28.1 | 0.022 |
| C-reactive protein (mg/l) | 4.0 ± 6.6 | 5.4 ± 5.3 | 7.7 ± 9.3 | <0.001 |
Results were expressed as mean ± SD unless otherwise noted. eGFR, estimated glomerular filtration rate; PAI-1, plasminogen activator inhibitor-1.
TABLE 2.
Baseline characteristics of the Strong Heart Family Study participants according to plasma plasminogen activator inhibitor-1 levels (2001–2003, N = 1502)
| Characteristics | Level of plasma PAI-1 (ng/ml) | P value for linear trenda | ||
|---|---|---|---|---|
| Low (5–32) | Intermediate (33–57) | High (58–441) | ||
| N | 508 | 453 | 541 | |
| Age (years) | 31.9 ± 13.0 | 33.9 ± 13.0 | 33.6 ± 12.2 | 0.044 |
| Male [n (%)] | 182 (35.83) | 189 (41.72) | 191 (35.30) | 0.835 |
| Current smoking [n (%)] | 175 (34.45) | 182 (40.18) | 239 (44.18) | 0.002 |
| Current drinking [n (%)] | 315 (62.01) | 292 (64.46) | 358 (66.17) | 0.189 |
| Obesity [n (%)] | 123 (24.21) | 202 (44.59) | 380 (70.24) | <0.001 |
| Dietary sodium (mg/day) | 3726.1 ± 3448.1 | 3993.2 ± 3593.7 | 3963.7 ± 4088.3 | 0.424 |
| SBP (mmHg) | 113.4 ± 10.9 | 116.0 ± 10.2 | 117.5 ± 10.5 | <0.001 |
| DBP (mmHg) | 71.7 ± 9.4 | 73.7 ± 8.6 | 75.1 ± 8.7 | <0.001 |
| BMI (kg/m2) | 26.6 ± 5.8 | 30.0 ± 6.8 | 34.1 ± 7.6 | <0.001 |
| Fasting plasma glucose (mg/dl) | 94.0 ± 31.6 | 98.7 ± 33.4 | 109.2 ± 45.5 | <0.001 |
| Total cholesterol (mg/dl) | 170.6 ± 30.1 | 177.6 ± 35.6 | 184.1 ± 36.6 | <0.001 |
| Triglycerides (mg/dl) | 113.8 ± 59.5 | 136.7 ± 84.5 | 185.6 ± 139.1 | <0.001 |
| LDL-cholesterol (mg/dl) | 91.7 ± 25.5 | 99.7 ± 30.0 | 101.5 ± 29.7 | <0.001 |
| HDL-cholesterol (mg/dl) | 56.3 ± 14.9 | 51.1 ± 12.3 | 46.9 ± 13.0 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 101.5 ± 22.8 | 100.4 ± 22.8 | 104.3 ± 22.2 | 0.084 |
| C-reactive protein (mg/l) | 4.3 ± 8.0 | 5.4 ± 8.6 | 7.0 ± 7.9 | <0.001 |
Results were expressed as mean ± SD unless otherwise noted. eGFR, estimated glomerular filtration rate; PAI-1, plasminogen activator inhibitor-1.
Using generalized estimating equation models to account for family relatedness among study participants.
Prospective association of plasma plasminogen activator inhibitor-1 with incident hypertension in the Strong Heart Study
Of the 1019 SHS participants, 305 developed new hypertension during a median 4.0 years of follow-up. The incidence density of hypertension was 75.1 per 1000 person-years. The prospective association of baseline plasma PAI-1 with incident hypertension in the SHS was shown in Table 3.
TABLE 3.
Prospective association between plasma plasminogen activator inhibitor-1 and risk of hypertension in American Indians
| Multivariate adjustedb | ||||
|---|---|---|---|---|
|
|
||||
| Plasma PAI-1 (ng/ml) | No. of incident hypertension | Incidence densitya | OR (95% CI) | P value |
| SHS participants (N = 1019) | ||||
| Continuous | ||||
| Log-transformed PAI-1 | 305 | 75.1 | 1.35 (1.06–1.72) | 0.014 |
| Categorical | ||||
| Low (<33) | 78 | 59.5 | 1.00 (reference) | – |
| Intermediate (33–57) | 112 | 79.9 | 1.46 (1.02–2.09) | 0.039 |
| High (≥58) | 115 | 85.3 | 1.63 (1.12–2.37) | 0.011 |
|
| ||||
| SHFS participants (N = 1502) | ||||
| Continuous | ||||
| Log-transformed PAI-1 | 258 | 31.2 | 1.41 (1.11–1.81) | 0.007 |
| Categorical | ||||
| Low (<33) | 58 | 20.6 | 1.00 (reference) | – |
| Intermediate (33–57) | 81 | 32.5 | 1.37 (0.90–2.08) | 0.133 |
| High (≥58) | 119 | 40.1 | 1.64 (1.08–2.50) | 0.023 |
CI, confidence interval; OR, odds ratio; PAI-1, plasminogen activator inhibitor-1; SHFS, the Strong Heart Family Study; SHS, the Strong Heart Study.
No. per 1000 person-years.
Adjusting for age, sex, study site, current smoking, current drinking, dietary sodium, obesity, fasting glucose, LDL cholesterol and HDL cholesterol, estimated glomerular filtration rate, C-reactive protein, and follow-up years.
Logistic regression using log-transformed PAI-1 as a continuous variable showed that a higher level of plasma PAI-1 was significantly associated with an increased risk of hypertension [odds ratio (OR) = 1.35, 95% confidence interval (CI): 1.06–1.72, P = 0.014], after adjusting for age, sex, study site, current smoking, current drinking, dietary sodium, obesity, fasting glucose, LDL-C, HDL-C, eGFR, C-reactive protein, and follow-up years. Results of logistic regression based on plasma PAI-1 tertiles (low vs. intermediate vs. high) detected an association in the same direction. Compared with participants with low PAI-1, those with intermediate and high PAI-1 had a 46% (OR = 1.46, 95% CI: 1.02–2.09, P = 0.039) and 63% (OR = 1.63, 95% CI: 1.12–2.37, P = 0.011) increased risk for developing hypertension, respectively.
Results of replication in the Strong Heart Family Study
During a median 5.3 years of follow-up, 258 of the 1502 SHFS participants developed new hypertension. The incidence density of hypertension was 31.2 per 1000 person-years. The prospective association of baseline plasma PAI-1 with incident hypertension in SHFS participants was similar to that observed in SHS participants (Table 3).
After adjusting for age, sex, study site, current smoking, current drinking, dietary sodium, obesity, fasting glucose, LDL-C, HDL-C, eGFR, C-reactive protein, and follow-up years, logistic regression using log-transformed PAI-1 as the independent variable revealed a significant and positive association between plasma PAI-1 and risk of hypertension (OR = 1.41, 95% CI: 1.11–1.81, P = 0.007). Logistic regression using categorical PAI-1 level (low vs. intermediate vs. high) as the independent variable found that participants with high PAI-1 had a 64% extra risk for developing hypertension (OR = 1.64, 95% CI: 1.07–2.50, P = 0.023) but those with intermediate PAI-1 had a marginally increased risk for incident hypertension (OR = 1.37, 95% CI: 0.90–2.08) compared with those with low PAI-1.
Results of sensitivity analysis
The use of more restrictive definitions for incident hypertension did not attenuate the observed association between plasma PAI-1 and hypertension risk. Specifically, the multivariate-adjusted ORs for hypertension restricted to individuals who initiated treatment during follow-up, or had a SBP at least 160 mmHg, or DBP at least 95 mmHg at the end of follow-up for higher log-transformed PAI-1 were 1.45 (95% CI: 1.09–1.94, P = 0.012) in SHS participants and 1.40 (95% CI: 1.05–1.87, P = 0.023) in SHFS participants (Supplementary Table S1, http://links.lww.com/HJH/A769). Additional adjustment for baseline SBP did not change the association between plasma PAI-1 and incident hypertension in both cohorts (Supplementary Table S2, http://links.lww.com/HJH/A769). Exclusion of participants with prevalent diabetes did not change the observed association between plasma PAI-1 and incident hypertension in SHFS participants, but results became statistically nonsignificant in SHS participants (Supplementary Table S3, http://links.lww.com/HJH/A769). Adding plasma PAI-1 to the prediction model did not significantly increase prediction performance for hypertension compared with the model including conventional risk factors only in either cohort (Supplementary Fig. S1, http://links.lww.com/HJH/A769).
DISCUSSION
In two longitudinal cohorts of American Indians participating in the SHS (1993–1995) and the SHFS (2001–2003), we found that baseline plasma PAI-1 significantly predicted risk of hypertension, independent of many known risk factors. Specifically, a higher level of plasma PAI-1 was associated with over 35% increased risk of developing hypertension in this high-risk population. Our results suggest that plasma PAI-1 may contribute to the development of hypertension through pathways beyond traditional risk factors.
The observed association between plasma PAI-1 and incident hypertension in our study is in line with previous animal and human studies. For example, experimental research has demonstrated that PAI-1 deficiency induced by either PAI-1 gene knockout [2,5,7] or pharmacological inhibition [6,7] was protective against the development of hypertension, cardiac hypertrophy, and vascular fibrosis in mice. In human study, individuals carrying the 4G allele of PAI-1 gene exhibited a higher level of plasma PAI-1 than those carrying the 5G allele [28], and carrying two copies of the 4G allele was associated with higher SBP, DBP, and mean arterial BP in a population comprising 194 elderly women [29]. The association of plasma PAI-1 with BP or hypertension was also reported in several other cross-sectional and case–control epidemiological studies [8–14]. Although these studies clearly demonstrated an association between plasma PAI-1 and hypertension, the prospective relationship between plasma PAI-1 and incident hypertension in large, community-based longitudinal cohorts is still lacking. We are aware of only one prospective study examining the association of plasma PAI-1 with incident hypertension among 1456 European whites in the Framingham Offspring Study [17]. The authors reported that participants with higher level of plasma PAI-1 was associated with an increased risk of hypertension (OR = 1.28, 95% CI: 1.05– 1.57 for per SD increment). Our study confirms this association with similar effect size and provides initial evidence for a potential prognostic effect of plasma PAI-1 on hypertension risk in American Indians. The prospective relationship between plasma PAI-1 and incident hypertension was also in agreement with previous studies demonstrating that plasma PAI-1 significantly predicted risk of arterial stiffness [15], atherosclerosis [16], and cardiovascular events [3], all of which have been associated with hypertension.
Plasma PAI-1 was found to be associated with several risk factors of hypertension, such as obesity [30,31], insulin resistance [31,32], and inflammation [33]. However, the observed association between plasma PAI-1 and hypertension in our study was unlikely to be confounded by these chronic conditions because these factors were adjusted in our statistical models. Moreover, our sensitivity analysis indicated that the observed prospective association was robust to potential confounding by possible misclassification of incident hypertension and prevalent diabetes (at least in the SHFS). Although the mechanism underlying the relationship between PAI-1 and hypertension remains to be determined, it is possible that PAI-1 may affect hypertension risk through its effect on vascular remodeling [2], because overproduction of PAI-1 has been shown to accelerate perivascular and medial fibrosis through plasmin inhibition and inhibition of vascular smooth muscle cell migration in mice [34]. Vascular remodeling may increase peripheral resistance and reduced elasticity, which in turn contribute to risk for hypertension [35].
Although we identified that baseline plasma PAI-1 significantly predicted the risk of incident hypertension, it did not significantly increase the prediction performance over conventional risk factors. Thus, the clinical utility of PAI-1 in risk prediction for hypertension warrants further investigation. Despite that a number of PAI-1 inhibitors have been used to examine the pharmacological effect of PAI-1 inhibition in vitro and in vivo [36], large-scale clinical trials are clearly needed to evaluate the potential clinical utility of PAI-1 inhibitors in disease prevention or intervention.
To our knowledge, our study represents the first to examine the prospective association of plasma PAI-1 with incident hypertension in American Indians. The strengths of this study include replication in two longitudinal cohorts and comprehensive adjustments of many known risk factors including dietary sodium. However, our study also has several limitations. First, although our statistical analyses adjusted for many covariates, we cannot entirely exclude residual confounding by unknown and/or unmeasured factors. Second, all our study participants are American Indians whose cardiovascular health profiles may be different from those with other racial/ethnic backgrounds. Thus, the generalizability of our results to other populations is uncertain.
In summary, our study demonstrates that elevated plasma PAI-1 is significantly associated with an increased risk of future hypertension among American Indians independent of many known risk factors. More research is needed to establish the causality between PAI-1 and hypertension risk.
Supplementary Material
Acknowledgments
We would like to thank for the Strong Heart Study participants, Indian Health Service facilities, and participating tribal communities for their extraordinary cooperation and involvement, which has contributed to the success of the Strong Heart Study. The views expressed in this article are those of the authors and do not necessarily reflect those of the Indian Health Service.
The study was supported by NIH grants R01DK091369, K01AG034259, and R21HL092363 and cooperative agreement grants U01HL65520, U01HL41642, U01HL41652, U01HL41654, and U01HL65521.
Abbreviations
- CVD
cardiovascular diseases
- eGFR
estimated glomerular filtration rate
- HDL-C
HDL cholesterol
- IQR
interquartile range
- LDL-C
LDL cholesterol
- MCMC
the Markov Chain Monte Carlo
- OR
odds ratio
- PAI-1
plasminogen activator inhibitor-1
- ROC
the receiver operating characteristic
- SHFS
the Strong Heart Family Study
- SHS
the Strong Heart Study
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.De Taeye B, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol. 2005;5:149–154. doi: 10.1016/j.coph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation. 2001;104:839–844. doi: 10.1161/hc3301.092803. [DOI] [PubMed] [Google Scholar]
- 3.Johansson L, Jansson JH, Boman K, Nilsson TK, Stegmayr B, Hallmans G. Tissue plasminogen activator, plasminogen activator inhibitor-1, and tissue plasminogen activator/plasminogen activator inhibitor-1 complex as risk factors for the development of a first stroke. Stroke. 2000;31:26–32. doi: 10.1161/01.str.31.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 5.Kaikita K, Schoenhard JA, Painter CA, Ripley RT, Brown NJ, Fogo AB, Vaughan DE. Potential roles of plasminogen activator system in coronary vascular remodeling induced by long-term nitric oxide synthase inhibition. J Mol Cell Cardiol. 2002;34:617–627. doi: 10.1006/jmcc.2002.2001. [DOI] [PubMed] [Google Scholar]
- 6.Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, et al. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nomega-nitro-l-arginine methyl ester-induced hypertension and vascular senescence. Circulation. 2013;128:2318–2324. doi: 10.1161/CIRCULATIONAHA.113.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, et al. Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol. 2005;25:365–371. doi: 10.1161/01.ATV.0000152356.85791.52. [DOI] [PubMed] [Google Scholar]
- 8.Gavriilaki E, Gkaliagkousi E, Nikolaidou B, Triantafyllou G, Chatzopoulou F, Douma S. Increased thrombotic and impaired fibrinolytic response to acute exercise in patients with essential hypertension: the effect of treatment with an angiotensin II receptor blocker. J Hum Hypertens. 2014;28:606–609. doi: 10.1038/jhh.2014.18. [DOI] [PubMed] [Google Scholar]
- 9.Poli KA, Tofler GH, Larson MG, Evans JC, Sutherland PA, Lipinska I, et al. Association of blood pressure with fibrinolytic potential in the Framingham offspring population. Circulation. 2000;101:264–269. doi: 10.1161/01.cir.101.3.264. [DOI] [PubMed] [Google Scholar]
- 10.Eliasson M, Jansson JH, Nilsson P, Asplund K. Increased levels of tissue plasminogen activator antigen in essential hypertension. A population-based study in Sweden. J Hypertens. 1997;15:349–356. doi: 10.1097/00004872-199715040-00005. [DOI] [PubMed] [Google Scholar]
- 11.Makris T, Stavroulakis G, Papadopoulos D, Paizis I, Krespi P, Tsoukala C, et al. White coat hypertension and haemostatic/fibrinolytic balance disorders. Eur Cytokine Netw. 2006;17:137–141. [PubMed] [Google Scholar]
- 12.Coban E, Ozdogan M. The plasma levels of plasminogen activator inhibitor-1 in subjects with white coat hypertension. Int J Clin Pract. 2004;58:541–544. doi: 10.1111/j.1368-5031.2004.00119.x. [DOI] [PubMed] [Google Scholar]
- 13.Tiryaki O, Buyukhatipoglu H, Usalan C. Plasma plasminogen activator inhibitor 1 (PAI-1) and P-selectin levels in urgent hypertension: effect of single dose captopril and nifedipine on fibrinolytic activity. Clin Exp Hypertens. 2010;32:347–351. doi: 10.3109/10641961003628478. [DOI] [PubMed] [Google Scholar]
- 14.Armas-Hernandez MJ, Hernandez-Hernandez R, Armas-Padilla MC, Sosa-Canache B, Cammarata R, Pacheco B, et al. Fibrinolytic system in normotensive subjects and hypertensive patients. Am J Ther. 2007;14:177–182. doi: 10.1097/01.pap.0000249923.06373.95. [DOI] [PubMed] [Google Scholar]
- 15.Lieb W, Larson MG, Benjamin EJ, Yin X, Tofler GH, Selhub J, et al. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation. 2009;119:37–43. doi: 10.1161/CIRCULATIONAHA.108.816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahran M, Nasr FM, Metwaly AA, El-Sheikh N, Khalil NS, Harba T. The role of hemostatic factors in atherosclerosis in patients with chronic renal disease. Electron Physician. 2015;7:1270–1276. doi: 10.14661/1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TJ, Gona P, Larson MG, Levy D, Benjamin EJ, Tofler GH, et al. Multiple biomarkers and the risk of incident hypertension. Hypertension. 2007;49:432–438. doi: 10.1161/01.HYP.0000256956.61872.aa. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control & Prevention (CDC) Diagnosed diabetes among American Indians and Alaska Natives aged <35 years – United States, 1994–2004. MMWR Morb Mortal Wkly Rep. 2006;55:1201–1203. [PubMed] [Google Scholar]
- 19.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 20.Sakata K, Shirotani M, Yoshida H, Urano T, Takada Y, Takada A. Differential effects of enalapril and nitrendipine on the fibrinolytic system in essential hypertension. Am Heart J. 1999;137:1094–1099. doi: 10.1016/s0002-8703(99)70368-6. [DOI] [PubMed] [Google Scholar]
- 21.Best LG, North KE, Tracy RP, Lee ET, Howard BV, Palmieri V, Maccluer JW. Genetic determination of acute phase reactant levels: the Strong Heart Study. Hum Hered. 2004;58:112–116. doi: 10.1159/000083032. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Jablonski KA, Resnick HE, Jain AK, Jones KL, Gottlieb AM, et al. Alcohol intake and glycemia in American Indians: the Strong Heart Study. Metabolism. 2003;52:129–135. doi: 10.1053/meta.2003.50020. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, Mete M, Desale S, Fretts AM, Cole SA, Best LG, et al. Leukocyte telomere length and ideal cardiovascular health in American Indians: the Strong Heart Family Study. Eur J Epidemiol. 2017;32:67–75. doi: 10.1007/s10654-016-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 28.Sartori MT, Vettor R, De Pergola G, De Mitrio V, Saggiorato G, Della Mea P, et al. Role of the 4G/5G polymorphism of PaI-1 gene promoter on PaI-1 levels in obese patients: influence of fat distribution and insulin-resistance. Thromb Haemost. 2001;86:1161–1169. [PubMed] [Google Scholar]
- 29.Bjorck HM, Eriksson P, Alehagen U, De Basso R, Ljungberg LU, Persson K, et al. Gender-specific association of the plasminogen activator inhibitor-1 4G/5G polymorphism with central arterial blood pressure. Am J Hypertens. 2011;24:802–808. doi: 10.1038/ajh.2011.63. [DOI] [PubMed] [Google Scholar]
- 30.Eksteen P, Pieters M, de Lange Z, Kruger HS. The association of clot lysis time with total obesity is partly independent from the association of PAI-1 with central obesity in African adults. Thromb Res. 2015;136:415–421. doi: 10.1016/j.thromres.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 31.Lalic K, Jotic A, Rajkovic N, Singh S, Stosic L, Popovic L, et al. Altered daytime fluctuation pattern of plasminogen activator inhibitor 1 in type 2 diabetes patients with coronary artery disease: a strong association with persistently elevated plasma insulin, increased insulin resistance, and abdominal obesity. Int J Endocrinol. 2015;2015:390185. doi: 10.1155/2015/390185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, et al. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283:221–228. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 33.Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemost. 2008;100:969–975. [PubMed] [Google Scholar]
- 34.Ghosh AK, Bradham WS, Gleaves LA, De Taeye B, Murphy SB, Covington JW, Vaughan DE. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation. 2010;122:1200–1209. doi: 10.1161/CIRCULATIONAHA.110.955245. [DOI] [PubMed] [Google Scholar]
- 35.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3 Pt 2):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 36.Van De Craen B, Declerck PJ, Gils A. The biochemistry, physiology and pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2012;130:576–585. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.