Abstract
This study seeks to investigate the possible protective role of the methanol extract of Piper guineense seeds against CCl4-induced hepatotoxicity in an animal model. Hepatotoxicity was induced by administering oral doses of CCl4 (1.2 g/kg bw) three times a week for three weeks. Group 1 (Control) and Group 2 (CCl4) were left untreated; Piper guineense (PG; 400 mg/kg bw) was administered to Group 3 (T1) by oral gavage for 14 days prior to the administration of CCl4 and simultaneously with CCl4; PG (400 mg/kg bw) was administered simultaneously with CCl4 in Group 4 (T2); and Livolin forte (20 mg/kg bw) was administered simultaneously with CCl4 in Group 5 (T3), the standard drug group. The administration of CCl4 induces histopathological alteration in the liver, with concomitant increased activities of serum hepatic marker enzymes associated with increased levels of lipid peroxidation. Similarly, there was decrease in non-enzymatic (reduced glutathione) and enzymatic antioxidants (glutathione S-transferase), superoxide dismutase, and catalase. An elevation in serum triglyceride and total cholesterol levels was noticed along with decreased levels of serum total protein. Treatment with PG 400 mg/kg bw exhibited excellent modulatory activity with respect to the different parameters studied by reversing all the above-mentioned biochemical changes significantly in the experimental animals. These results suggest that PG offered protection comparable to that of Livolin forte with better efficacy when pre-treated with 400 mg/kg bw 14 days prior to CCl4-exposure.
Keywords: antioxidant enzymes, carbon tetrachloride, lipid peroxidation, liver toxicity, Piper guineense
1. Introduction
The current increase in the incidence and prevalence of liver diseases remain a major global health burden. Chronic liver diseases have been identified as the fifth most common cause of death in the United Kingdom, as well as accounting for about 4.8% deaths among American Indians and Alaska Natives [1,2]. The liver plays an essential role in metabolism and the excretion of various therapeutic agents and xenobiotics from the body [3,4]. There has been no definite therapeutic agent for a total cure of liver diseases; documented scientific evidence suggests that most of the available therapeutic agents facilitate the healing or regeneration of the liver [5]. The liver itself has an exceptional regenerative capacity after any cellular injury as a result of xenobiotic exposure (toxic chemicals, ethanol, pesticides, drugs, CCl4 etc.). To ascertain the hepatoprotective effect of drugs/molecules during drug screening, Carbon tetrachloride (CCl4) induced hepatic injury remains an excellent commonly used model [3].
Exposure to xenobiotics, especially CCl4, has been implicated in the etiology and progression of oxidative stress and inflammation in a number of acute and chronic disorders. Direct exposure to CCl4 triggers cascades of events. CCl4 elicits hepatotoxicity through its biotransformation to trichloromethyl radicals (CCl3) or trichloroperoxyl radicals (CCl3O2), produced by the mixed-function cytochrome P450 oxygenase system of the endoplasmic reticulum, which induces lipid peroxidation of membranes that leads to hepatocellular damage [6,7]. Antioxidant therapies have been shown to play a crucial role in maintaining balance and preventing the body from several diseases caused by the overproduction of free radicals. Naturally, antioxidant therapies act as free radical scavengers, thereby inhibiting lipid peroxidation [8].
Medicinal plants are believed to be a rich source of antioxidants. Added to this, they are a cost effective and potent alternative with few and transient side effects in the treatment and management of liver disorders, when compared with the existing conventional therapeutic drugs available in the market [9,10,11,12]. Piper guineense Schum. and Thom. (Piperaceae), widely consumed as a spice in West Africa (commonly called African black pepper; with indigenous names such as Iyere in Yoruba and Uziza in Ibo), contain various biological and pharmacological properties. These properties have therapeutic potential in the prevention and management of hepatotoxicity. P. guineense has been recently reported to contain free radical scavengers such as polyphenols, alkaloids, flavonoids, saponins, tannins, and glycosides in appreciable quantities [13]. Previous studies have also reported on the anti-parasitic, antifungal, anticonvulsant, antimicrobial, anti-inflammation activities, as well as the anti-schistosomal and hepatoprotective activities, of Piper guineense [13,14,15]. In view of this, the present study was designed to assess the possible protective mechanism of Piper guineense against CCl4-induced hepatotoxicity and its potential role in the inhibition of oxidative stress in an animal model.
2. Materials and Methods
2.1. Chemicals
All the chemicals and reagents (analytical grade and the purest quality available) were obtained from Sigma Chemical (St. Louis, MO, USA) and Merck (Zug, Germany), while all kits were Randox assay kits.
2.2. Plant Material and Extract Preparation
Fresh Piper guineense seeds were obtained from a local vendor in Ibadan, Nigeria. The identification and authentication (voucher No. FHI 107745) were previously carried out by Nwozo and colleagues, 2012 [13]. One kilogram of the seeds was coarsely powdered using an electric blender and macerated with 80% methanol (200 g/1000 mL) with intermittent stirring for 72 h. The methanol extract was filtered, and the filtrate was concentrated in a rotary evaporator to obtain a (brown) crude extract, which was stored in a clean sample bottle at 4 °C. The yield of the extract was 132.74 g (13.27% w/w) of the starting material. The portions of the extract used for this experiment were weighed and reconstituted in distilled water daily, just before administration to the animals.
2.3. Animals and Experimental Design
Thirty male Wistar albino rats (weighing 180–200 g) obtained from the Animal House of the Department of Biochemistry, College of Sciences, Afe Babalola University, were randomly divided into five groups and classified into control (negative control), CCl4 (positive control), T1 (pre-treatment), T2 (post-treatment), and T3 (standard drug). The animals were acclimatized for fourteen days before commencing the experiment and were maintained on standard feed and water ad libitum. The animals were kept in well-ventilated cages at room temperature (28–30 °C) and under controlled light cycles (12 h light/12 h dark). All procedures were carried out in compliance with the protocols approved by the Animal Ethical Committee of Afe Babalola University (ABUAD-SCIREC04/14/02/069).
Group 1 (Control) served as the control. Group 2 (CCl4) served as the CCl4 control. Group 3 (T1) received CCl4 and Piper guineense extract (400 mg/kg) for 14 days prior to the administration of CCl4 and simultaneously with CCl4 administration. Group 4 (T2) received CCl4 and Piper guineense extract simultaneously (400 mg/kg), while Group 5 (T3), the standard drug group, received CCl4 and Livolin forte (20 mg/kg bw) simultaneously. The Piper guineense extracts (400 mg/kg bw) and Livolin forte (20 mg/kg bw) were given by oral gavage [16]. CCl4 (1.2 g/kg bw) was administered orally three times a week (Mondays, Wednesdays, and Fridays) for three weeks to induce toxicity.
2.4. Blood and Tissue Collection
The experiment lasted for 21 days, and, on day 22, the overnight fasted animals were sacrificed. Blood samples were collected and allowed to coagulate at room temperature. The clear, non-haemolysed supernatant sera were quickly removed and stored at −20 °C for subsequent analysis. Liver samples were rapidly expunged, weighed, and washed in ice-cold 1.15% KCl solution; they were homogenized in 56 mM Tris-HCl buffer (pH 7.4), comprised of 1.15% KCl, and then centrifuged at 10,000× g for 15 min. The supernatant was carefully separated and kept till required for analysis. A histological assessment of the liver was carried out under a light microscope. Small liver samples were fixed in 10% normal saline and then dehydrated and paraffin-embedded for histological assessment.
2.5. Biochemical Assays
The ferric reducing antioxidant potential (FRAP) and oxygen radical absorption capacity (ORAC) activities of the methanol extract was determined using the methods described by Benzie and Strain, 1996 [17], and Ou and co-workers, 2001 [18], respectively. Briefly, 10 μL of the diluted plant extracts was mixed with 300 μL FRAP reagent in a 96-well clear plate. The FRAP reagent was a mixture (10:1:1, v/v/v) of acetate buffer (300 mM, pH 3.6), tripyridyl triazine (TPTZ) (10 mM in 40 mM HCl), and FeCl3⋅6H2O (20 mM). After incubation at room temperature for 30 min, the plate was read at a wavelength of 593 nm in a Multiskan Spectrum plate reader (51118650, Thermo Fisher Scientific, Waltham, MA, USA). Ascorbic acid (AA) was used as the standard, and the results were expressed as μmol AAE/g sample, while the ORAC assay was carried out using a 96-well microplate using a Fluorescence plate reader (51118650, Thermo Fisher Scientific, Waltham, MA, USA). The reaction consisted of 12 μL of diluted aqueous plant extracts and 138 μL of fluorescein (14 μM), which was used as a target for free radical attack. The reaction was initiated by the addition of 50 μL 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH), and the fluorescence was (emission 538 nm, excitation 485 nm) recorded every 1 min for 2 h. Trolox was used as the standard, and the results were expressed as μmol/g sample. All determinations were done in triplicates. The activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) were determined according to the method of Reitman and Frankel, 1957 [19]. The serum total proteins concentration was assessed using the method described by Henry, 1964 [20]; total cholesterol and triglycerides were evaluated by routine enzymatic methods using Randox commercial kits.
The extent of lipid peroxidation was measured by the malondialdehyde (MDA) content. This was assessed using the thiobarbituric acid method described by Varshney and Kale, 1990 [21]. The levels of reduced glutathione (GSH) in the liver homogenate were estimated using the method described by Beutler et al., 1963 [22]. Glutathione-S-transferase (GST) activity was determined according to Habig et al., 1974 [23]. The level of superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich, 1972 [24]. Catalase (CAT) activity was determined by adopting the method described by Sinha, 1972 [25].
2.6. Statistical Analysis
SPSS version 10.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The values were presented as the means ± SD of different groups. The data were analysed with one-way analysis of variance (ANOVA). The results were considered statistically significant when p < 0.05.
3. Results
3.1. Antioxidant Capacity of Piper guineense Methanol Extract
Before embarking on the animal experiment, the FRAP and ORAC activities of the methanol extract were determined. The FRAP and ORAC (693.01 ± 0.28 μmol AAE/mL and 207.41 ± 0.16 μmol TE/mL) values obtained compare well with the values documented in the literature (Table 1).
Table 1.
The antioxidant capacity of Piper guineense methanol extract.
| Parameter | Value in Piper guineense |
|---|---|
| Ferric reducing antioxidant potential (FRAP) (μmol AAE/mL) | 693.01 ± 0.28 |
| Oxygen radical absorption capacity (ORAC) (μmol TE/mL) | 207.41 ± 0.16 |
3.2. Effect of Piper guineense on Indices of Hepatotoxicity
The current work showed that CCl4 treatment markedly increased the activities of liver serum biomarker (AST, ALT, and ALP) enzymes (Table 2). The concomitant administration of PG extract with CCl4 showed significant restoration in serum biomarker activities.
Table 2.
The effect of Piper guineense on indices of hepatotoxicity.
| Groups | Aspartate Aminotransferase (AST) Activity (U/L) | Alanine Aminotransferase (ALT) Activity (U/L) | Alkaline Phosphatase (ALP) Activity (U/L) |
|---|---|---|---|
| Control | 34.84 ± 1.12 b | 21.35 ± 0.18 b | 109.87 ± 0.43 a |
| CCl4 | 56.12 ± 1.08 a | 29.09 ±0.12 a | 145.88 ± 0.38 b |
| T1 | 42.55 ± 1.51 a,b | 21.87 ± 0.13 | 117.72 ± 0.08 |
| T2 | 51.96 ± 1.36 a | 23.90 ± 0.71 | 138.09 ± 0.18 a |
| T3 | 44.70 ± 1.34 a,b | 23.34 ± 0.16 | 119.09 ± 0.51 |
The values shown are mean ± S.D. (n = 6). Mean differences are significant (p < 0.05) when compared with: a control group, b CCl4 only.
3.3. Influence of Piper guineense on Serum Total Protein, Total Cholesterol and Triglyceride Levels
The serum level of total protein was remarkably reduced, whereas the total cholesterol and triglyceride levels were elevated in the in CCl4-treated rats compared to the control group (Table 3). Treatment with PG extract attenuated the deleterious effect of CCl4 on serum total protein, total cholesterol, and triglyceride.
Table 3.
Effect of Piper guineense on serum total protein, total cholesterol, and triglyceride levels.
| Groups | Total Protein (g/dL) | Total Cholesterol (mg/dL) | Triglycerides (mg/dL) |
|---|---|---|---|
| Control | 29.26 ± 0.23 b | 142.44 ± 0.26 b | 46.64 ± 0.14 a |
| CCl4 | 23.49 ± 0.36 a | 261.22 ± 0.20 a | 98.42 ± 0.82 b |
| T1 | 34.36 ± 0.36 a,b | 189.66 ± 0.02 a,b | 49.19 ± 0.11 |
| T2 | 27.51 ± 0.14 | 223.03 ± 0.16 a,b | 63.98 ± 0.28 a |
| T3 | 32.77 ± 0.27 | 201.86 ± 0.23 a,b | 51.21 ± 0.24 a,b |
The values shown are mean ± S.D. (n = 6). Mean differences are significant (p < 0.05) when compared with: a control group, b CCl4 only.
3.4. Effect of Piper guineense on Assessment of Oxidative Stress
Summarized in Table 4 is the modulatory effect of PG extract on oxidative stress in CCl4-treated rats. The administration of CCl4 significantly induced lipid peroxidation and diminished the status of the enzymatic and non-enzymatic antioxidants in the liver. The administration of PG extract adequately inhibited the stimulation of lipid peroxidation and compensated the diminished antioxidant status.
Table 4.
The effect of Piper guineense on the assessment of oxidative stress.
| Groups | Lipid Peroxidation (LPO) | Reduced Glutathione (GSH) | Glutathione-S-Transferase (GST) | Superoxide Dismutase (SOD) | Catalase (CAT) |
|---|---|---|---|---|---|
| Control | 13.35 ± 1.45 b | 41.21 ± 0.41 b | 19.12 ± 1.89 b | 7.09 ± 0.11 b | 24.21 ± 0.71 b |
| CCl4 | 28.40 ± 1.82 a | 28.26 ± 0.26 a | 12.61 ± 1.08 a | 3.41 ± 0.26 a | 16.79 ± 0.86 a |
| T1 | 19.14 ± 0.89 a | 37.56 ± 0.43 b | 18.86 ± 1.13 b | 7.23 ± 0.16 b | 23.61 ± 0.53 b |
| T2 | 23.92 ± 1.14 a | 35.05 ± 0.33 b | 15.08 ± 1.79 | 5.91 ± 0.13 | 19.20 ± 0.29 |
| T3 | 17.69 ± 1.86 a | 37.34 ± 0.21 b | 19.59 ± 1.46 b | 7.01 ± 0.08 b | 21.44 ± 0.12 b |
The values shown are mean ± S.D. (n = 6). Mean differences are significant (p < 0.05) when compared with: a control group, b CCl4 only. Units: LPO (unit/mg protein), GSH (µg/mL), GST (µm/min/mg protein), SOD (units/mg protein), CAT (units/mg protein).
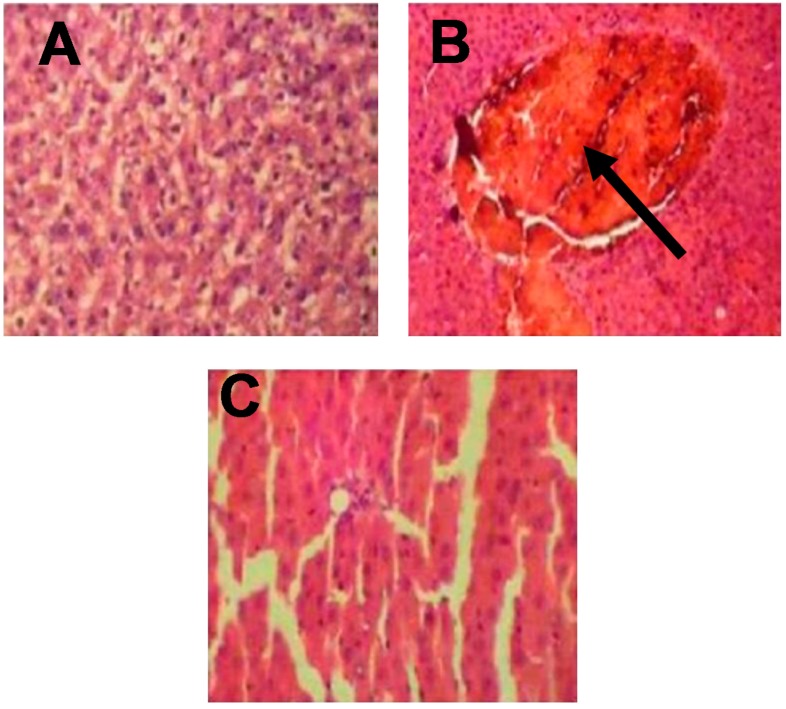
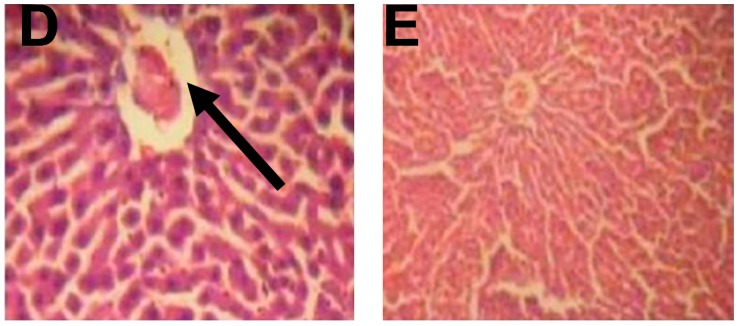
3.5. Histological Examination of Rat Livers
The results obtained from the histological studies of the liver tissue showing histopathological alterations are presented in Figure 1. The control (negative control) group showed normal hepatic architecture with no lesions or abnormalities, while the CCl4 (positive control) group showed congestion in the central vein associated with the infiltration of inflammatory cells. In the T1 (pre-treatment) group, mild hepatocytes necrosis and mononuclear cellular infiltration was observed, while, in the T2 (post-treatment) group, mild portal tract and lobular chronic inflammation with focal hepatocyte destruction were seen. Inflammatory cell infiltration and hepatocytes necrosis were hardly detected in the T3 (standard drug) group.
Figure 1.
Histological examination of rat livers stained with hematoxylin and eosin (H&E). (A) Control: showing normal hepatic architecture with no lesions or abnormalities; (B) CCl4: showing congestion in the central vein associated with the infiltration of inflammatory cells; (C) T1: showing mild hepatocytes necrosis and mononuclear cellular infiltration; (D) T2: showing mild portal tract and lobular chronic inflammation with focal hepatocyte destruction; (E) T3: showing that improved hepatic architecture, inflammatory cell infiltration, and hepatocytes necrosis were hardly detected (×400).
4. Discussion
There has been increased focus on the chemopreventive potential and possible mechanisms of action of plant derived foods in the form of nutraceuticals in the prevention, treatment, and management of liver diseases caused by oxidative stress due to indiscriminate exposure to xenobiotics in our environment. Various studies have shown that the formation of reactive intermediates (CCl3 or CCl3O2 radicals) as a result of CCl4-intoxication plays an essential role in the emergence of heaptocellular injury [26]. Before embarking on the animal experiment, the FRAP and ORAC activities of the methanol extract were determined. It is interesting to note that the values obtained are relatively high and compare well with the values documented in literature; this may be an indication that the plant extracts possess antioxidant potential. The higher the absorbance value of the extract, the higher is its antioxidant capacity [27,28].
The results from a pilot study conducted at Afe Babalola University (unpublished data) revealed that the methanol extract was more effective than the aqueous and hexane extracts at the examined dose (400 mg/kg bw), with no significant sign of toxicity. In the present study, the exposure of animals to CCl4-treatment resulted in a remarkable increase in the activities of liver biomarker enzymes; notably, AST, ALT, and ALP. Clinically, this suggests that there is a certain degree of damage to the liver. CCl4-toxicity has been characterized by a loss of cell membrane integrity, increased permeability of the hepatocytes membrane, and cellular leakage [29,30,31]. Treatment with PG extract (400 mg/kg) exhibited significant restoration of serum markers in the pre-treatment and post-treatment groups when compared with the standard drug group (Livolin forte; 20 mg/kg bw), indicating its protection against CCl4-induced hepatocellular injury.
In the present study, it is obvious that the reduction witnessed in the level of serum total protein in CCl4-treated rats can be attributed to the initial damage in hepatocytes, causing reduction in mixed function oxidases activity and inhibition of protein metabolism in the liver. The elevation in the serum total cholesterol and triglyceride levels in CCl4-treated rats may have resulted from the increase in oxidative stress, which enhances deterioration in hepatic function and the accumulation of lipids, leading to a fatty liver due to the failure of their secretory mechanisms [32,33,34,35]). Treatment with PG extract (pre-treatment and post-treatment) attenuated the deleterious effect of CCl4 on serum total protein, total cholesterol, and triglyceride and caused a subsequent recovery towards normalization.
The present increase in lipid peroxidation and the consequent suppression of antioxidant activities in CCl4-treated rats has been well documented in the literature as an indication of oxidative stress [36,37,38]. Free radical mediated lipid peroxidation via the formation of reactive intermediate (CCl3 or CCl3O2 radicals) is the major mechanism of hepatocellular injury by CCl4 [36,39]. The cell protects itself from oxidative damage by recruiting reduced glutathione (GSH) and scavenging enzymes such as CAT, SOD, glutathione peroxidase (GPx), and GST as first-line cellular defenses in response to oxidative challenges in order to protect cellular integrity. Suppression in the status of these enzymatic and non-enzymatic antioxidants in the hepatic homogenates is an indication of the overwhelming effect of CCl4 on their normal redox state within the cells [40,41,42,43].
Accordingly, the oral administration of PG extract significantly repressed the CCl4-induced elevation of lipid peroxidation and also enhanced the status of these enzymatic and non-enzymatic antioxidants. This complemented the previously identified protective roles (the ability to restore hepatocytes and protect membrane integrity, as well as the ability to reduce the leakage of the hepato-specific enzymes) of PG extracts [13]. The mode of action of P. guineense extracts in this experiment is similar to that of Livolin forte [44]. The histological examination of the liver samples strongly supports the modulatory role of P. guineense extract in CCl4-induced toxicity.
5. Conclusions
The results obtained from this study demonstrate that extracts from P. guineense possess antioxidant and hepatoprotective properties comparable to that of Livolin forte, with better efficacy when pre-treated with 400 mg/kg bw 14 days prior to CCl4-exposure in an animal model. We propose that the possible mechanism by which PG brought about the observed changes in the present study may be due to the synergistic interactions of its bioactive components. Therefore, as we investigate nature’s recipes for health, P. guineense is a candidate with promising therapeutic potential.
Acknowledgments
The research reported in this manuscript was supported by the South African Medical Research Council through funding received from the South African National Treasury. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the South African Medical Research Council. More so, the first author acknowledges Afe Babalola University, for awarding a research fellowship for educational advancement, as well as the University of Zululand Research Committee.
Author Contributions
Babatunji Emmanuel Oyinloye and Abidemi Paul Kappo conceived and designed experiments; contributed reagents, consumables, and analysis tool; and wrote the submitted manuscript. Foluso Oluwagbemiga Osunsanmi, Basiru Olaitan Ajiboye, and Oluwafemi Adeleke Ojo performed the experiments, analysed the data, and wrote the initial draft of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 2.Suryaprasad A., Byrd K.K., Redd J.T., Perdue D.G., Manos M.M., McMahon B.J. Mortality caused by chronic liver disease among American Indians and Alaska Natives in the United States, 1999–2009. Am. J. Public Health. 2014;104:S350–S358. doi: 10.2105/AJPH.2013.301645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang H.L., Wang Y.J., Zhang Q.Y., Liu B., Wang F.Y., Li J.J., Zhu R.Z. Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J. Gastroenterol. 2012;18:6605. doi: 10.3748/wjg.v18.i45.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan R.A., Khan M.R., Sahreen S. CCl4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complement. Altern. Med. 2012;12:178. doi: 10.1186/1472-6882-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huo H.Z., Wang B., Liang Y.K., Bao Y.Y., Gu Y. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int. J. Mol. Sci. 2011;12:6529–6543. doi: 10.3390/ijms12106529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Rasheed N.M., Al-Rasheed N.M., Faddah L.M., Mohamed A.M., Mohammad R.A., Al-Amin M. Potential impact of silymarin in combination with chlorogenic acid and/or melatonin in combating cardiomyopathy induced by carbon tetrachloride. Saudi J. Biol. Sci. 2014;21:265–274. doi: 10.1016/j.sjbs.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahashwan S., Hassan M.H., Aly H., Ghobara M.M., El-Beshbishy H.A., Busati I. Crocin mitigates carbon tetrachloride-induced liver toxicity in rats. J. Taibah. Univ. Sci. 2015;10:140–149. doi: 10.1016/j.jtumed.2014.09.003. [DOI] [Google Scholar]
- 8.Lee J., Koo N., Min D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp. Rev. Food Sci. Food Saf. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 9.Ortega-Ramirez L.A., Rodriguez-Garcia I., Leyva J.M., Cruz-Valenzuela M.R., Silva-Espinoza B.A., Gonzalez-Aguilar G.A., Siddiqui M., Ayala-Zavala J.F. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: A hypothesis. J. Food Sci. 2014;79:R129–R137. doi: 10.1111/1750-3841.12341. [DOI] [PubMed] [Google Scholar]
- 10.Guan Y.S., He Q. Plants consumption and liver health. Evid. Based Complement. Altern. Med. 2015:824185. doi: 10.1155/2015/824185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013;10:210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey N., Meena R.P., Rai S.K., Pandey-Rai S. Medicinal plants derived nutraceuticals: A re-emerging health aid. Int. J. Pharm. Biosci. 2011;2:419–441. [Google Scholar]
- 13.Nwozo S.O., Ajagbe A.A., Oyinloye B.E. Hepatoprotective effect of Piper guineense aqueous extract against ethanol-induced toxicity in male rats. J. Exp. Integr. Med. 2012;2:71–76. doi: 10.5455/jeim.241111.or.016. [DOI] [Google Scholar]
- 14.Ali F.U., Ominyi M.C., Nwankwo O.V.U., Ibiam U.A., Ogbanshi M.E. Comparative effects of ethanolic extract of Gongronema latifolium and Piper guineense on blood electrolytes in ethanol exposed Wistar rats. Biochem. Anal. Biochem. 2015;4:179. [Google Scholar]
- 15.Uhegbu F.O., Imo C., Ugbogu A.E. Effect of aqueous extract of Piper guineense seeds on some liver enzymes, antioxidant enzymes and some hematological parameters in Albino rats. Int. J. Plant Sci. Ecol. 2015;1:167–171. [Google Scholar]
- 16.Oyinloye B.E., Adenowo A.F., Osunsanmi F.O., Ogunyinka B.I., Nwozo S.O., Kappo A.P. Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. SpringerPlus. 2016;5:641. doi: 10.1186/s40064-016-2228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Ou B., Hampsch-Woodill M., Prior R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 19.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transminases. Am. J. Clin. Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Henry R.J. Clinical Chemistry, Principles and Technics. 2nd ed. Harper and Row; New York, NY, USA: 1974. [Google Scholar]
- 21.Varshney R., Kale R.K. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990;58:733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 22.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 23.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 24.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 25.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 26.Sahreen S., Khan M.R., Khan R.A. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement. Altern. Med. 2011;11:48. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudonne S., Vitrac X., Coutiere P., Woillez M., Mérillon J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 28.Avasthi A.S., Jawaid S.A., Jain S., Bhatnagar M., Purkayastha S., Ghosal S. Free radical scavenging and antioxidant impact of Indian medicinal plant extracts on H2O2 mediated oxidative stress on human erythrocytes. Adv. J. Phytomed. Clin. Ther. 2014;2:1052–1069. [Google Scholar]
- 29.AshganA A., Haiam M., Aboul-Ela E.A., Ahemd E.M.K., Ola K.S. Hepatoprotective, DNA damage prevention and antioxidant potential of Spirulina platensis on CCl4-induced hepatotoxicity in mice. Am. J. Biomed. Res. 2015;3:29–34. [Google Scholar]
- 30.Forouzandeh H., Azemi M.E., Rashidi I., Goudarzi M., Kalantari H. Study of the protective effect of Teucrium polium L. extract on acetaminophen-induced hepatotoxicity in mice. Iran. J. Pharm. Res. 2013;12:123–129. [PMC free article] [PubMed] [Google Scholar]
- 31.Nasir A., Abubakar M.G., Shehu R.A., Aliyu U., Toge B.K. Hepatoprotective effect of the aqueous leaf extract of Andrographis paniculata Nees against carbon tetrachloride–induced hepatotoxicity in rats. Niger. J. Basic Appl. Sci. 2013;21:45–54. doi: 10.4314/njbas.v21i1.7. [DOI] [Google Scholar]
- 32.Essawy A.E., Abdel-Moneim A.M., Khayyat L.I., Elzergy A.A. Nigella sativa seeds protect against hepatotoxicity and dyslipidemia induced by carbon tetrachloride in mice. J. Appl. Pharm. Sci. 2012;2:21–25. doi: 10.7324/JAPS.2012.21004. [DOI] [Google Scholar]
- 33.Ahsan R., Islam K.M., Musaddik A., Haque E. Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride induced hepatotoxicity in albino rats. Glob. J. Pharmacol. 2009;3:116–122. [Google Scholar]
- 34.Anusha M., Venkateswarlu M., Prabhakaran V., Taj S.S., Kumari B.P., Ranganayakulu D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011;43:563–567. doi: 10.4103/0253-7613.84973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan X., Hussain F.N., Iqbal J., Feuerman M.H., Hussain M.M. Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4-induced steatosis. J. Biol. Chem. 2007;282:17078–17089. doi: 10.1074/jbc.M701742200. [DOI] [PubMed] [Google Scholar]
- 36.Ritesha K.R., Suganyaa A., Dileepkumar H.V., Rajashekar Y., Shivanandappa T.A. Single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain. Toxicol. Rep. 2015;2:891–895. doi: 10.1016/j.toxrep.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulfraz M., Ahamd D., Ahmad M.S., Qureshi R., Mahmood R.T., Jabeen N., Abbasi K.S. Effect of leaf extracts of Taraxacum officinale on CCl4 induced Hepatotoxicity in rats, in vivo study. Pak. J. Pharm. Sci. 2014;27:825–829. [PubMed] [Google Scholar]
- 38.Gnanaraj C., Haque A.E., Iqbal M. The chemopreventive effects of Thysanolaena latifolia against carbon tetrachloride (CCl4)-induced oxidative stress in rats. J. Exp. Integr. Med. 2012;2:345–355. doi: 10.5455/jeim.030912.or.045. [DOI] [Google Scholar]
- 39.Moreira P.R., Maioli M.A., Medeiros H.C., Guelfi M., Pereira F.T., Mingatto F.E. Protective effect of bixinon carbon tetrachloride induced hepatotoxicity in rats. Biol. Res. 2014;47:49. doi: 10.1186/0717-6287-47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang B.Y., Zhang X.Y., Guan S.W., Hua Z.C. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules. 2015;20:12250–12265. doi: 10.3390/molecules200712250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thanh T.B., Thanh H.N., Minh H.P.T., Le-Thi-Thu H., Ly H.D.T., Duc L.V. Protective effect of Tetracera scandens L. leaf extract against CCl4-induced acute liver injury in rats. Asian Pac. J. Trop. Biomed. 2015;5:221–227. doi: 10.1016/S2221-1691(15)30009-5. [DOI] [Google Scholar]
- 42.Hismiogullari A.A., Hismiogullari S.E., Karaca O., Sunay F.B., Paksoy S., Can M., Kus I., Seyrek K., Yavuz O. The protective effect of curcumin administration on carbon tetrachloride (CCl4)-induced nephrotoxicity in rats. Pharmacol. Rep. 2015;67:410–416. doi: 10.1016/j.pharep.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Kartik R., Rao C.V., Trivedi S.P., Pushpangadan P., Reddy G.D. Amelioration effects against N-nitrosodiethylamine and CCl4-induced hepatocarcinogenesis in Swiss albino rats by whole plant extract of Achyranthes aspera. Indian J. Pharmacol. 2010;42:370–375. doi: 10.4103/0253-7613.71921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olukiran O.S., Akomolafe R.O., Bamitale K.D., Ajayi A.O., Okonji R.E., Bejide R.A. Protective and curative effects of Livolin forte® on carbon tetrachloride-induced liver damage in Wistar rats. J. Exp. Integr. Med. 2014;4:57–65. doi: 10.5455/jeim.310813.or.088. [DOI] [Google Scholar]