Abstract
Greater attention is currently being paid to the relationship between indoor environment and childhood allergies, however, the lack of reliable data and the disparity among different areas hinders reliable assessment of the relationship. This study focuses on the effect of indoor pollution on Chinese schoolchildren and the relationship between specific household and health problems suffered. The epidemiological questionnaire survey and the field measurement of the indoor thermal environment and primary air pollutants including CO2, fine particulate matter (PM2.5), chemical pollutants and fungi were performed in six Chinese cities. A total of 912 questionnaires were eligible for statistical analyses and sixty houses with schoolchildren aged 9–12 were selected for field investigation. Compared with Chinese national standards, inappropriate indoor relative humidity (<30% or >70%), CO2 concentration exceeding 1000 ppm and high PM2.5 levels were found in some monitored houses. Di(2-ethylhexyl) phthalate (DEHP) and dibutyl phthalate (DBP) were the most frequently detected semi-volatile organic compounds (SVOCs) in house dust. Cladosporium, Aspergillus and Penicillium were detected in both indoor air and house dust. This study indicates that a thermal environment with CO2 exceeding 1000 ppm, DEHP and DBP exceeding 1000 μg/g, and high level of PM2.5, Cladosporium, Aspergillus and Penicillium increases the risk of children’s allergies.
Keywords: indoor pollution, schoolchildren, allergic diseases, field investigation, fine particulate matter (PM2.5), semi-volatile organic compounds (SVOCs), fungi
1. Introduction
The indoor environment is of great importance to human health as in modern society people spend approximately 90% of their time indoors, especially at their own homes [1,2,3]. In general, children spend more time indoors than adults. In such a condition, and aligned with the accelerated growth of their more immature organs which achieve higher metabolic rates [4,5], children are more susceptible to the effects of indoor environmental exposure. With the rapid modernization and urbanization of China, the prevalence of childhood allergic diseases such as asthma, rhinitis and eczema have correspondingly been rapidly increasing over the recent decades [6,7]. A large-scale survey on childhood asthma in China conducted by the National Cooperative Group indicated that the prevalence of asthma between the ages of 0–14, increased from 1.5% in 2000 to 3.02% in 2010, hence an increase of 101.3% [8,9]. Of further interest and importance in this area is the emerging and accumulating evidence indicating that both genetic and environmental factors play important roles in the etiology of allergic diseases. Results suggest that genetic factors are not the only ones for the rapid and increasingly common manifestations of allergic diseases,which makes it feasible that environmental factors are important contributors.
A growing number of studies suggest that dwelling environment, lifestyles and indoor air pollution contribute to the development of allergic disease [10]. Recent meta-analysis [11,12] concluded that living or attending schools near high traffic density roads with higher levels of motor vehicle air pollutants increased the incidence and prevalence of childhood asthma. Sun et al. [13] declared that the lifestyle and home environment exposure undergone by children between 1–8 years are important asthma and allergy risk factors in Northeast Texas. Likewise, indoor dampness is considered a risk factor for children’s health. Bornehag et al. [14] found that indoor moisture characterization increased the risk of respiratory symptoms (cough, wheezing and asthma) for children aged 1–6. In addition, a series of studies have concluded that high indoor humidity is strongly related to childhood bronchial obstruction, rhinitis and respiratory symptoms [15,16,17,18].
Carbon dioxide (CO2) is a natural product of human metabolism, but high CO2 levels can result in unhealthy symptoms such as sore throat, irritated nose/sinus, combined mucous membrane, tight chest and wheezing [19]. Indoor chemical pollutants are mainly emitted from building materials. Some evidence has clearly indicated that the increasing risk of allergy, asthma and airway inflammation in children were due to exposure to formaldehyde (HCHO) [20,21]. Additionally, volatile organic compound (VOC) emissions from building materials were related to asthma, wheezing and allergies in children [22]. Even at low concentrations of the pollutant benzene, an essential VOC category, has been proven to have an association with childhood respiratory health [23]. Fine particulate matter (PM2.5) is one of the major environmental concerns in China due to the prevalence of haze. Its indoor exposure could result in the increase of cumulative incidence of the lower respiratory symptoms, and also be related to allergic inflammation and persistent asthma in children [24,25]. As an indoor biological pollutant, fungi are ubiquitous in children’s indoor environments, with exposure to fungal spores being a widely recognized inducing factor as regards respiratory diseases and atopic dermatitis [26,27,28]. Additionally, fungi have been reported as a positive association of symptoms of respiratory and allergic diseases such as wheezes, daytime breathlessness, allergic rhinitis, hypersensitivity pneumonitis and doctor-diagnosed asthma [29,30]. Cladosporium is a significant allergen and has an adverse effect on asthmatics or respiratory diseases [31]. Penicillium is known to produce mycotoxins that can affect human health [32]. House dust includes mites, pollen, mold and semi-volatile organic compounds (SVOCs). The increasing awareness, in recent years about the effect of such as the above, has alerted the effect of the exposure to SVOCs in house dust on childhood asthma or allergies. It has been suggested that children are more vulnerable than adults, to phthalate exposure through household floor dust, and, in addition, it was becoming obvious that the levels of SVOCs in house dust were, infact, associated with the prevalence of allergic rhinitis, conjunctivitis, and atopic dermatitis in children [33]. Di(2-ethylhexyl) phthalate (DEHP), as one of the most common SVOCs, is suspected as a risk factor in childhood asthma and allergic diseases [34,35]. A recent negative consideration in house dust, has been the presence of dibutyl phthalate (DBP), which is thought to have possible associations with previously diagnosed eczema and eye symptoms [36].
In China, an increasing interest in the association between indoor pollution and allergic diseases in children has developed. The China, Child, Homes, Health project conducted a cross-sectional questionnaire survey to examine the associations between in-house environmental factors and the prevalence of asthma and allergies among children aged 3–6 [37]. Deng et al. [38,39] reported that both prenatal and early postnatal exposure to various indoor environmental factors correlated with preschool children suffering from asthma, allergy and various infections. The findings of Liu et al. [40] indicated that culturable fungi and particle concentrations showed strong positive correlations with preschool children’s respiratory health. Mi et al. [41] monitored CO2, nitrogen dioxide (NO2), ozone (O3) and formaldehyde in naturally ventilated schools for pupils (13–14 years old) in Shanghai. Zhan et al. [42,43] investigated indoor NO2, CO2, sulfur dioxide (SO2) and microbial components in settled dust in junior high schools in Taiyuan. However, the above studies: (1) focused mostly on preschool children, and rarely involved children of school age, (2) involved only one or several indoor pollutants, and (3) rarely simultaneously examined these pollutant levels in the home environment and in relation to children’s health.
Schoolchildren aged from 10 to 12 years old are considered to be on the brink of adolescence. Their exposure to such as pollutants is higher than that of adults as their intake is greater per unit body weight via the multiplicity of pathways. Thus, in residential buildings, which are the most likely centres for children’s daily after school activities, chronic air pollutant exposure, in such circumstances as described above, may provide a negative health impact, particularly for the more clinically susceptible. Further data was collected via a systematical investigative study, composed of a questionnaire survey and field investigation related to environmental conditions was then conducted in several major Chinese cities, including Harbin, Dalian [44], Beijing [45], Shanghai [46], Wuhan [47] and Changsha [48]. This study included two stages: (1) cross-sectional questionnaire survey concerning residential characteristics and lifestyles, (2) a detailed field investigation of the environmental conditions of the schoolchildren’s households. The six cities chosen for this study are in different climate zones [49]: Harbin is located in severe cold zone, Beijing and Dalian are located in the cold zone, while Shanghai, Wuhan and Changsha are located in hot summer and cold winter zone.
The objectives of this study were: (1) to inspect environmental conditions in children’s households including building characteristics and lifestyles; (2) to quantitatively assess the levels of indoor pollutants and personal exposure; (3) to compare the differences between the indoor pollution experienced by (a) healthy and (b) unhealthy children’s households and (4) to identify risk factors associated with allergic diseases experienced by children.
2. Materials and Methods
2.1. Consent
Informed consent was obtained from children’s parents before survey and measurement. All involved families agreed to participate in the survey and part of them allowed us to conduct the measurement in their houses.
2.2. QuestionnaireSurvey
During 2012–2013, a questionnaire survey was performed in the 4th- and 5th-grade school pupils. Participants were from the affiliated primary schools of Harbin Institute of Technology, Dalian University of Technology, Beijing University of Technology, Shanghai Jiao Tong University, Huazhong University of Science and Technology, and Hunan University. Approximately 1200 questionnaires were distributed to children’s families in the above mentioned schools. Questionnaires were filled out by the parents of children in accord with their actual situation. Finally, a total of 912 questionnaires were eligible for inclusion in the analysis.
The interview sheet referred to the American Thoracic Society-Division of Lung Disease (ATS-ELD) questionnaires. The contents mainly included [50]: (1) general information of the child; (2) dwelling environment, including residential location, surroundings of household, house size and so on; (3) equipment in everyday use, including heating, cooling, ventilation and the associated degree of humidity, dryness, condensation/mold/visible flow; (4) lifestyle habits, such as feeding style, smoking, the presence of pets, washing frequency of children’s quilts and (5) the specific health status of children and family members and the subsequent allergic symptoms. The questionnaire includes about 80questions and some of them were listed as follows:
“Eczema in the last 2 years”: Has your child had doctor-diagnosed eczema in the last 2 years? (yes vs. no)
“Living near high traffic density roads”: Are you living near high traffic density roads? (yes vs. no)
“Condensation on windows”: Did condensation or moisture occur on the inside or at the bottom of windows(windowpanes) in the child’s bedroom? (yes vs. no)
“Visible mold”: Have you noticed any visible mold on the floor, walls or ceiling in the child’s bedroom? (yes vs. no)
“Visible water flow”: Have you noticed any visible water flow on the floor, walls or ceiling in the child’s bedroom? (yes vs. no)
“Breast feeding”: Has your child been breastfeed for more than 6 months since their birth? (yes vs. no)
“Would you like to cooperate with the detailed measurement about indoor air pollutants in your house? (yes vs. no)”
2.3. Field Measurement
Based on the surveyed health conditions, sixty participants (ten children from each city) were selected from the volunteers and detailed field measurements, as indicated above, were conducted in their houses. The sixty children were divided into two groups. Thirty with one or more doctor-diagnosed respiratory and allergic symptoms during the past 2 years, such as wheezing, breathlessness, eczema, urticaria, seasonal or allergic rhinitis, formed Group A. The remaining thirty children who over the last 2 years, had not experienced symptoms listed above, formed Group B.
The field measurements were carried out in the living rooms and bedrooms of each child during the winter of 2013. The measurements taken were as follows: (1) two-week monitoring of air temperature, relative humidity (RH) and CO2 level; (2) measurement of PM2.5 mass concentration; (3) test of HCHO, acetaldehyde, and total volatile organic compounds (TVOCs) in air; (4) identification of airborne fungi in air and settled fungi in house dust; (5) detection of SVOCs in house dust. Measurements were not taken during “cooking periods” as indoor PM2.5 level and carbonyls could be elevated during this period. Additionally, the residents were told not to clean the houses intentionally and keep the indoor environment in a daily state.
2.3.1. Monitoring of Air Temperature, RH and CO2 Concentration
The data logger with temperature and humidity sensors (TR-72Ui, T&D Corp., Matsumoto, Japan) was used for monitoring air temperature and RH at 10 min intervals of for 14 days. In each house, the instruments were located at three positions: at heights of 0.1 m and 1.1 m above the floor in the living room, and at a height of 1.1 m above the floor in the child’s bedroom. Care was taken to avoid the direct influence of both solar radiation and indoor heat sources on the sensors. The CO2 level in the living room was measured at an interval of 5 min for 14 days. The data logger with CO2 sensor (MCH-383SD, Lutron, Taipei, Taiwan) was placed at a suitable location on the floor, to avoid the direct influence of the occupants’ exhalation.
2.3.2. Monitoring of PM2.5 Level
An aerosol monitor (TSI 8354 DustTrak, Shoreview, MN, USA) was applied for measuring PM2.5 concentrations in the living room, child’s bedroom and outdoor air on the open balcony. The instrument was installed approximately 1.1 m above the floor to avoid the influence of floor dust and that of human respiration. The PM2.5 concentrations were recorded every second of three minutes and the mean values of 180 records were taken as the local PM2.5 level.
2.3.3. Sampling and Analysis of HCHO, Acetaldehyde and VOCs
Portable air samplers fitted with a mini pump (SIBATA MP-300, Saitama, Japan) were used to simultaneously sample air at a height of approximately 1.1 m in three areas: above the floor in the living room, the child’s bedroom and outdoors in each selected house. The sampling rate was set to 1 L/min and lasting for 30 min. Air samples for HCHO and acetaldehyde were contained in active gas tubes (SIBATA, DNPH active gas tube), and those for VOCs were collected in solid-phase samplers (SIBATA, charcoal tube standard). As soon as the sampling was completed, the tube was tightly plugged and sealed in an attached bag, specially designed to preserve the active gas tube. HCHO and acetaldehyde were analyzed by the high performance liquid chromatography (HPLC) method proposed by the Japanese Standard Association [51], and VOCs were analyzed by the gas chromatography/mass spectrometry (GC/MS) methods by Jis [52].
2.3.4. Sampling and Analysis of SVOCs in House Dust
House dust for SVOCs detection was gathered within the filter paper (2V, Whatman, Freiburg, Germany) by using a vacuum cleaner (PV-H23, Hitachi, Tokyo, Japan). The house dust samples were collected for 2 min from a 1 m2 floor area of the living room and child’s bedroom, and were analyzed by thermal desorption-gas chromatography/mass spectrometry (TD-GC/MS) [52].
2.3.5. Sampling and Analysis of Airborne and Settled Fungi
Airborne fungi were sampled with a one-stage impact or air sampler (BIO SAMP MBS-1000, Midori Anzen Co., Tokyo, Japan) by using the multi-orifice sieve impact method proposed by ISO 16000-18 [53]. Air samples (100 L) from the living room, child’s bedroom and outdoor were blown onto an agar plate containing dichloran-glycerol (DG 18) growth medium. After sampling, the agar plate was immediately covered with its lid and tightly sealed with sealing tape.
A vacuum cleaner (PV-H23, Hitachi Co.) was used to collect house dust samples from both the living room and the child’s bedroom. Dust sampling took place on a 1 m2 floor area for 2 min. After passing through a 300 μm mesh, coarse dust particles were removed and the finer dust particles were extracted, weighed and then suspended and agitated in a 10 mL sterilization solution. A 50 μL sample of the resulting suspension was then plated on a DG18 agar plate. The DG18 agar base was incubated at 25 °C for five days. The genera of fungi were determined by morphological analysis according to ISO 16000-17 [54]. The airborne fungi and settled fungi, repectively, were quantified with the number of colonies per cubic meter (CFU/m3) and per milligram of dust (CFU/mg).
2.4. Statistical Analysis
Multiple logistic regression models were used to evaluate the association between residential factors as presented above and childhood allergic disease, adjusting for gender, age, family history of allergies and environmental tobacco smoke exposure. Associations in the regression analysis were calculated as adjusted odds ratio (OR) with 95% confidence interval (95% CI). A p value less than 0.05 was considered statistically significant. The statistical analyses were performed by the IBM SPSS Statistics version 20.0 software (IBM International, Armonk, NY, USA). The Non-parametric Mann-Whitney U test was used to compare the differences between two groups of indoor pollutants found in the selected houses. Pearson’s correlation was employed for calculating the correlation coefficients among different air pollutants in houses.
3. Results
3.1. Questionnaire Data
The prevalence of allergies in children among six cities is shown for comparison in Table 1. Of the 912 children, 46.7% had an allergic symptom in the past, 41% had allergic diseases in the past, 36.4% had current allergies, and 32.6% exhibited evidence of pollinosis and 14% of eczema.
Table 1.
The prevalence of childhood allergies and symptoms among six Chinese cities.
| Symptoms | Prevalence (%) | ||||||
|---|---|---|---|---|---|---|---|
| Total | Harbin | Dalian | Beijing | Shanghai | Wuhan | Changsha | |
| (n = 912) | (n = 127) | (n = 120) | (n = 150) | (n = 205) | (n = 170) | (n = 140) | |
| Persistent cough | 2.2 | 2.36 | 0.8 | 2 | 2 | 2.4 | 3.6 |
| (n = 20) | (n = 3) | (n = 1) | (n = 3) | (n = 4) | (n = 4) | (n = 5) | |
| Persistent phlegm | 2 | 2.36 | 1.7 | 2 | 2 | 2.4 | 1.4 |
| (n = 18) | (n = 3) | (n = 2) | (n = 3) | (n = 4) | (n = 4) | (n = 2) | |
| Respiratory allergy | 8.8 | 5.51 | 9.2 | 0.7 | 12.2 | 12.9 | 10 |
| (n = 80) | (n = 7) | (n = 11) | (n = 1) | (n = 25) | (n = 22) | (n = 14) | |
| Pollinosis | 32.6 | 29.13 | 32.5 | 22 | 37.6 | 36.5 | 35 |
| (n = 297) | (n = 37) | (n = 39) | (n = 33) | (n = 77) | (n = 62) | (n = 49) | |
| Past allergic diseases | 41 | 43.31 | 38.3 | 28 | 50.7 | 43.5 | 37.9 |
| (n = 374) | (n = 55) | (n = 46) | (n = 42) | (n = 104) | (n = 74) | (n = 53) | |
| Current allergies | 36.4 | 32.28 | 35.8 | 25.3 | 42.9 | 39.4 | 38.6 |
| (n = 332) | (n = 41) | (n = 43) | (n = 38) | (n = 88) | (n = 67) | (n = 54) | |
| Past allergies | 46.7 | 43.31 | 55 | 41.3 | 50.7 | 50.6 | 37.9 |
| (n = 426) | (n = 55) | (n = 66) | (n = 62) | (n = 104) | (n = 86) | (n = 53) | |
| Eczema | 14 | 11.02 | 10.8 | 10.7 | 19 | 16.5 | 12.9 |
| (n = 128) | (n = 14) | (n = 13) | (n = 16) | (n = 39) | (n = 28) | (n = 18) | |
The associations between dwelling environment, lifestyles and childhood allergies were evaluated by multivariate logistic regression models and adjusted for potential confounding covariates including gender (boy vs. girl), age (9 years vs. 10 years vs. 11 years vs. 12 years), family history of allergies (yes vs. no) and environmental tobacco smoke exposure (Table 2). Due to the small number of children suffering from a persistent cough, persistent phlegm and respiratory allergies, multivariate analysis was performed, only, for other allergic diseases.
Table 2.
Multivariate logistic regression analysis of the dwelling environment, lifestyles and childhood allergic diseases in terms of adjusted odds ratio (OR) with 95% confidence interval (95% CI).
| Factors | Pollinosis n (OR, CI) | Current Allergies n (OR, CI) | Past Allergies n (OR, CI) | Past Allergic Diseases n (OR, CI) | Eczema n (OR, CI) |
|---|---|---|---|---|---|
| Living near high traffic density roads | - | 1.61 | - | - | - |
| (2.36, 5.41) * | |||||
| House redecoration | 3.52 | 3. 34 | - | - | - |
| (1.55, 7.97) * | (1.6, 6.83) * | ||||
| House size (<60 m2) | 2.31 | - | - | - | - |
| (1.29, 4.13) * | |||||
| Dampness | 4.31 | - | - | - | 4.07 |
| (2.34, 7.64) * | (1.96, 8.48) * | ||||
| Household pets | 3.39 | - | 4.42 | - | - |
| (1.22, 9.27) * | (1.59–12.27) * | ||||
| Light sleep | 1.61 | - | - | - | - |
| (5.39, 11.28) * | |||||
| Breast feeding | 0.21 | 0.19 | - | - | - |
| (0.1–0.43) ** | (0.1–0.42) ** | ||||
| Low cleaningfrequency of quilts | - | - | - | 2.08 | - |
| (1.15, 3.77) * |
* denotes p < 0.05, ** denotes p < 0.01.
Based on the above results, childhood pollinosis appears to significantly correlate with such factors as house redecoration activity conducted over the past 10 years, house sizes of less than 60 m2, indoor dampness (visible mold, condensation on windows or visible water flow on the floor, walls or ceiling), household pets feeding within the house and light sleep on the part of the child. In addition, living near highly dense traffic roads also possibly increases the risk of allergies in children.
Of interest is that history of allergies and allergic diseases were thought to have a respective correlation with the effects of pets feeding (as mentioned above), and such as low cleaning frequency of quilts. Indoor dampness was recognized as a risk factor for eczema in children. However, of interest is the finding that breast feeding (lasted for more than 6 months since their birth) provides a protective factor for children suffering from pollinosis and current allergies.
3.2. Indoor Thermal Environment
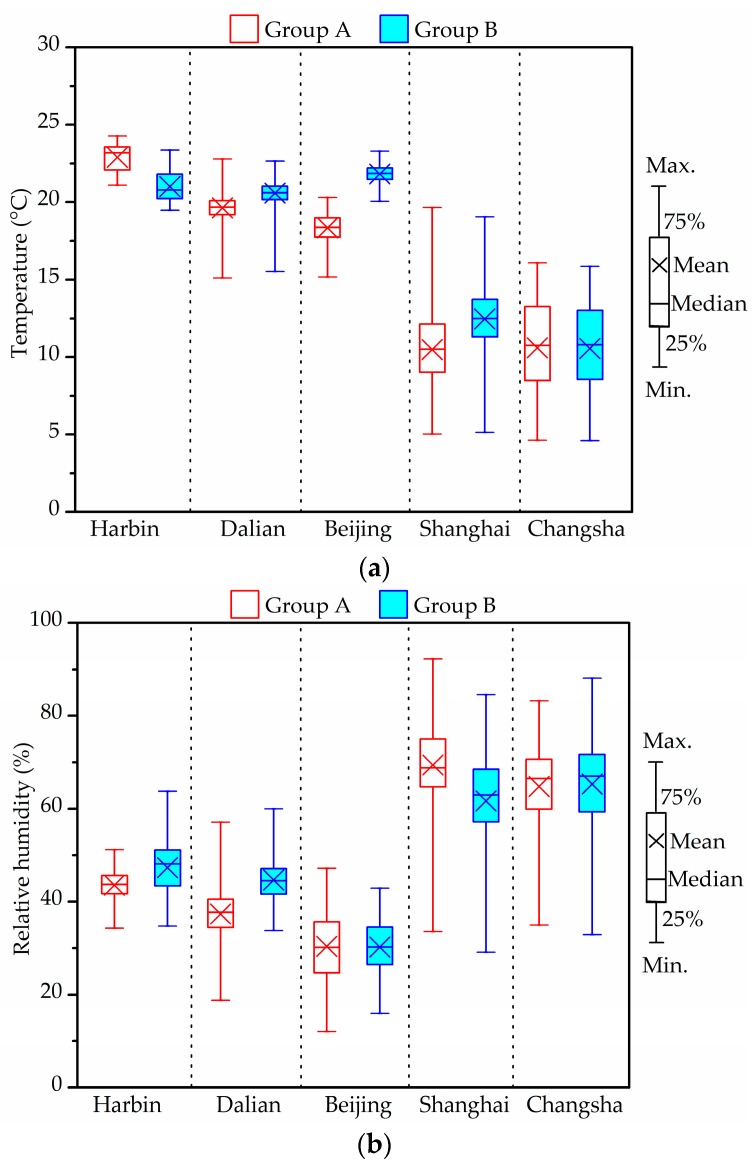
Figure 1 shows the distributions of the winter indoor temperature and RH experienced by the two groups of children, each group living in different cities. The values were the average value at the three measurement positions as mentioned above.
Figure 1.
Statistical box plots of indoor temperature (a) and relative humidity (RH) (b) in two groups in winter.
The characteristics of the thermal environments differ in relation to the individual specified conditions of each measurement group and city. In Harbin, the outdoor temperature varied mainly within the range 1.6 ± 2.4 °C, while RH varied within the range of 65.6 ± 18.2%. Indoor average temperatures, with an average of 22.9 °C in Group A and 21 °C in Group B, due to indoor heating, were much higher than those outdoors. Indoor average RH was less than 50%. Temperature and RH distributions in Dalian and Beijing were similar to those in Harbin. On the whole, the indoor temperature in the investigated houses of the above three cities met the standard value (16–24 °C). Compared with the Chinese indoor air quality (IAQ) standard value (30–60%), indoor RHs in both groups of Beijing and Group A in Dalian were relatively low.
Indoor air temperature in the houses in Shanghai (Group A: 10.5 ± 2.3 °C, Group B: 12.5 ± 2.2 °C) was slightly higher than the outdoor temperature (9.0 ± 3.4 °C). The average temperature in Groups A and B from Changsha were 10.6 ± 2.9 °C (outdoors: 8.3 ± 4.7 °C), thus no difference between the two groups. Indoor RH in the measured houses of these two cities, overall, varied in the range of 60–75%. The respective averages in Groups A/B were 69.4 ± 8.3%/61.7 ± 10.7% in Shanghai and 64.8 ± 9%/65.3 ± 10.7% in Changsha.
Houses in Harbin, Beijing and Dalian were installed with regional central heating, while those in Shanghai, Changsha and Wuhan depended on the use of individual space heaters in winter. However, and of interest, is that no heating systems or devices were in use during the experimental tasks conducted in the Shanghai, Wuhan and Changsha houses.
Yanagi et al. [55] indicated that relative humidity of 70% or more promoted the growth of mold. From the figure, indoor RHs in the Harbin, Dalian and Beijing homes were lower than 50%. Conversely, the percentage of RH exceeding 70% in Groups A and B from Changsha were 32.6% and 36.9%, respectively. In addition, the rates of RH ≥ 70% were higher than 60%, observed more frequently in Group A than in Group B in the Shanghai houses [46]. Dampness and indoor mold appeared to be serious risk factors for allergic symptoms among children.
3.3. Indoor CO2 Level
Table 3 shows the results of the indoor CO2 level tests, conducted in different cities in winter.
Table 3.
Indoor CO2 concentration in different cities in winter.
| Item | Groups | Harbin | Dalian | Beijing | Shanghai | Wuhan | Changsha |
|---|---|---|---|---|---|---|---|
| Average value (ppm) | A | 945 | 926 | 982 | 758 | 689 | 479 |
| B | 1098 | 1086 | 1227 | 701 | 808 | 496 | |
| Range (ppm) | A | 313–3083 | 333–3595 | 347–9540 | 327–2480 | 330–2158 | 316–1806 |
| B | 324–2975 | 279–6588 | 237–9794 | 306–3200 | 312–2970 | 294–2029 | |
| The ratio exceeding 1000 ppm | A | 43.4% | 34% | 40.8% | 17.1% | 10% | 0.7% |
| B | 55.1% | 32.5% | 43.5% | 14.1% | 38.2% | 1.8% |
From the above results, it is seen that over 32.5% of the measured data in Harbin, Dalian and Beijing are higher than 1000 ppm: factors in excess of which are considered a health risk according to the Chinese IAQ standard [56]. The maximum CO2 concentration found in Beijing, was 9754 ppm. The measured CO2 in houses in Harbin, Dalian and Beijing was greater than that in Shanghai, Wuhan and Changsha, possibly due to poor natural ventilation, as indicated above. The frequency of CO2 concentrations exceeding 1000 ppm in Changsha was in the area of 0.7% in Group A and 1.8% in Group B, hence lower than that in the other two Southern cities studied: Shanghai and Wuhan. As given above, it appears clear that poor household ventilation has the propensity to increase Children’s exposure to and hence impact of chemicals, particles and organisms, thus increasing the risk of such as asthma exacerbations and respiratory infections [57].
3.4. PM2.5 Level
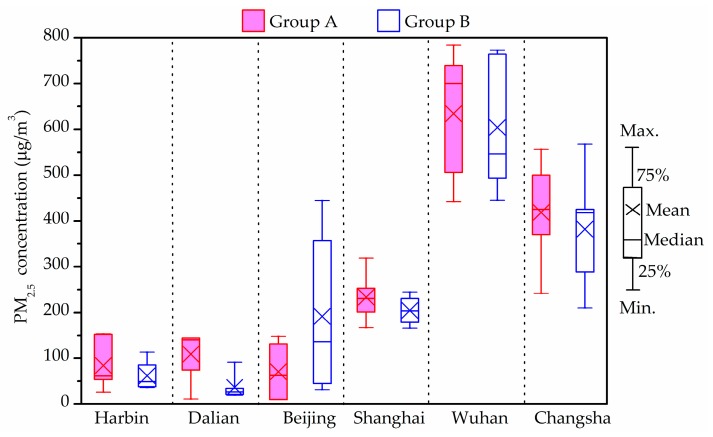
Figure 2 shows the indoor average PM2.5 concentrations in the investigated houses in winter. The PM2.5 levels in all measured households from Shanghai, Wuhan and Changsha exceeded the upper limit of the Chinese national standard [58], which suggests that the 24-h average PM2.5 concentration as 75 μg/m3 (Level-2). The indoor average PM2.5 concentrations of Group B in Harbin, Dalian and Group A in Beijing were 60 μg/m3, 36.5 μg/m3 and 71 μg/m3, respectively, hence satisfying the suggested limit regarding the protection of health. In addition, the indoor average PM2.5 concentrations of Group A except for Beijing conditions, were higher than those of Group B in the other five cities. However, and of interest, this difference showed no statistical significance (p > 0.05) between the two groups.
Figure 2.
Indoor average fine particulate matter (PM2.5) concentrations in winter.
The above statistics show that the PM2.5 concentrations in Wuhan and Changsha districts were higher than 500 μg/m3. According to the questionnaire responses and field investigation, it was found that some investigated houses were adjacent to roads with dense traffic [48]. Heavy vehicles emissions have been found to release serious PM2.5 pollution hence contaminating the adjacent outdoor air. The outdoor air was found to be the major PM2.5 source in those rooms with natural ventilation. Thus, combined with the heavy traffic pollution and poor outdoor air quality, the PM2.5 levels in indoor rooms could be extremely high in roadside households.
The indoor PM2.5 concentrations in Group B in Beijing were higher than those in Group A, however, the average indoor/outdoor (I/O) ratio of PM2.5 concentration in Group A (>1) was high than that in Group B (<1). A possible reason was the presence of stronger additional PM2.5 sources such as more smokers in Group A houses than that in houses of Group B.
3.5. Chemical Compound Concentration (HCHO, Acetaldehyde and TVOCs)
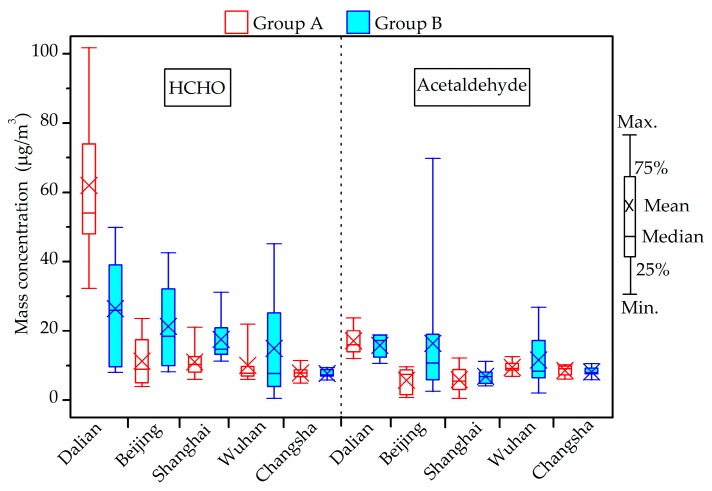
Figure 3 shows the concentrations of indoor chemical compounds, including HCHO and acetaldehyde in winter. The maximum HCHO concentration in all investigated houses was generally lower than the current guideline (100 μg/m3) provided by the Chinese national standards [56] except in one living room in Dalian (101.7 μg/m3). The highest concentration in Beijing, Shanghai, Wuhan and Changsha was 42.6 μg/m3, 31.1 μg/m3 [46], 45.2 μg/m3 [47] and 11.4 μg/m3, respectively, lower than half of the limit.
Figure 3.
Indoor formaldehyde (HCHO) and acetaldehyde concentrations in winter.
As for acetaldehyde, the current indoor air quality standards for residential buildings in China do not involve any reference limit. According to the Japanese national standard which suggests the upper acetaldehyde level of 48 μg/m3 [59], the maximum acetaldehyde concentration in all investigated houses was generally lower than this guideline, except in one living room in the houses of Beijing (69.8 μg/m3) [45]. Acetaldehyde levels in the measured houses of other cities varied from 0.5 to 26.9 μg/m3.
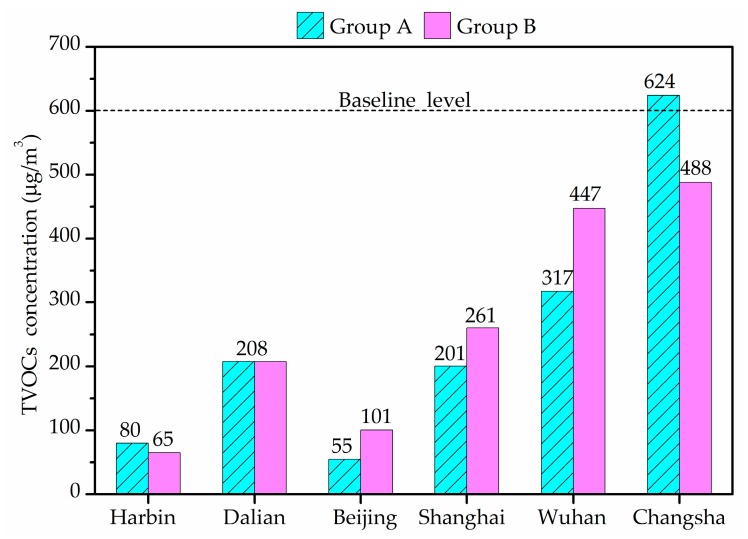
Figure 4 shows the indoor average TVOCs concentrations in winter. TVOCs concentrations revealed large differences between different cities houses. The average of TVOCs in Harbin and Beijing were less than 100 μg/m3 and those in Dalian, Shanghai and Wuhan were less than 600 μg/m3, the limit stipulated by the Chinese national standards [56]. However, the average indoor TVOCs concentration of Group A in Changsha was 624 μg/m3, exceeding the reference value. TVOCs concentration in Wuhan varied in a wide range and exceeded 600 μg/m3 in five air samples. There was no significant difference between Groups A and B.
Figure 4.
Indoor average total volatile organic compounds (TVOCs) concentrations in winter.
3.6. SVOCs Concentrations of Household Dust on Floors
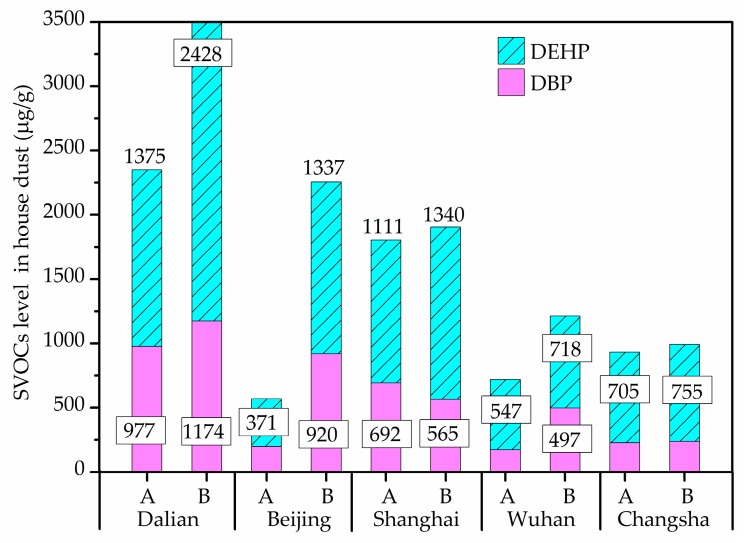
The most frequently detected components of SVOCs in house dust were found to be DEHP and DBP, with DEHP, alone, was present in all dust samples. Figure 5 shows the DEHP and DBP levels in all dust samples from different cities. In the revised standard Restriction of Hazardous Substances (RoHS) 2.0 (2015/863/EU) officially implemented in 2019 [60], DEHP and DBP, with the limit value of 1000 μg/g, were added. Compared with this guideline, DEHP and DBP in about 32.3% of the dust samples exceeded 1000 μg/g, with maximum concentrations detected in Dalian houses. However, the difference of DEHP and DBP between the two groups showed no statistical significance.
Figure 5.
Semi-volatile organic compounds (SVOCs) level in house dust in winter.
3.7. Fungi
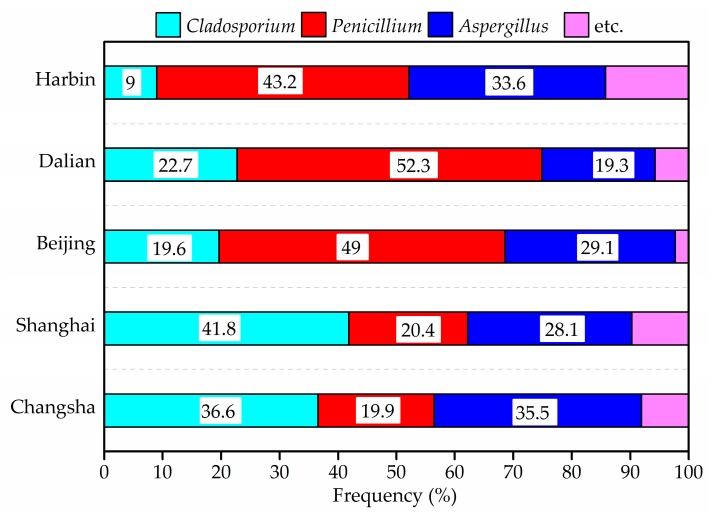
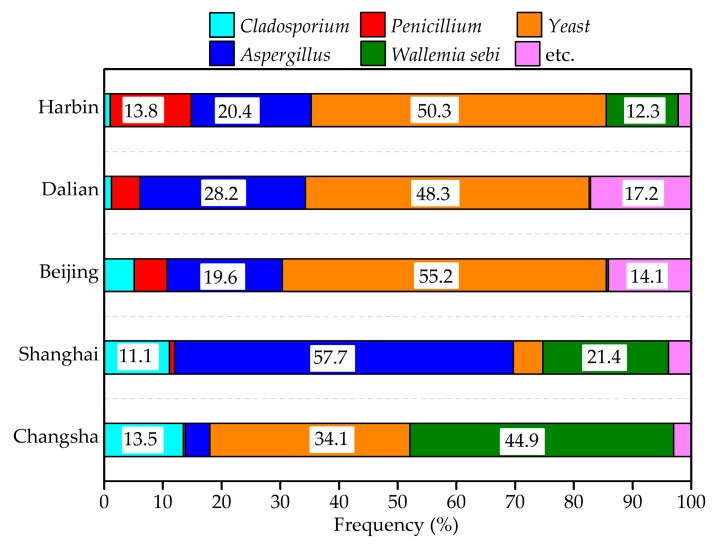
The results concerning the airborne fungi in the air and settled fungi in house dust are presented in Figure 6, Figure 7, Figure 8 and Figure 9. Three species of fungal allergen, i.e., Cladosporium, Aspergillus and Penicillium were the dominant species in the air and generally detected in all air samples. They accounted for 23.5%, 27.9% and 40.8%, respectively of indoor airborne fungi. As shown in Figure 7, the proportion of the main fungi species in air samples varied greatly from city to city. Penicillium in air samples from Harbin, Dalian and Beijing accounted for more than 43% of indoor airborne fungi, significantly higher than that of other species. Conversely, the percentage of Cladosporium in air samples from Shanghai and Changsha showed an increase.
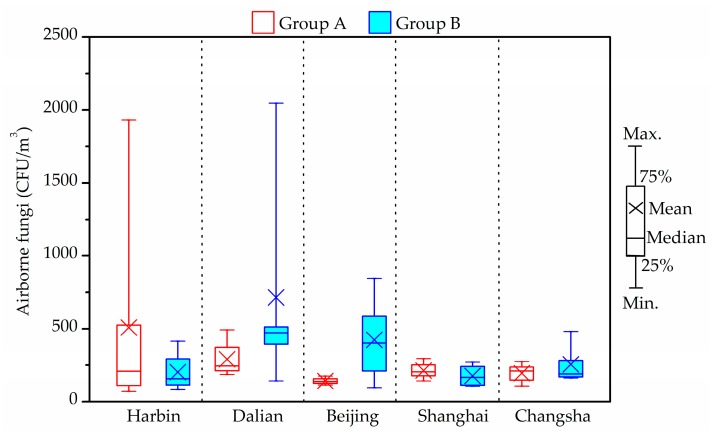
Figure 6.
Indoor average level of airborne fungi in air in winter.
Figure 7.
Frequency of airborne fungi species in indoor air in winter.
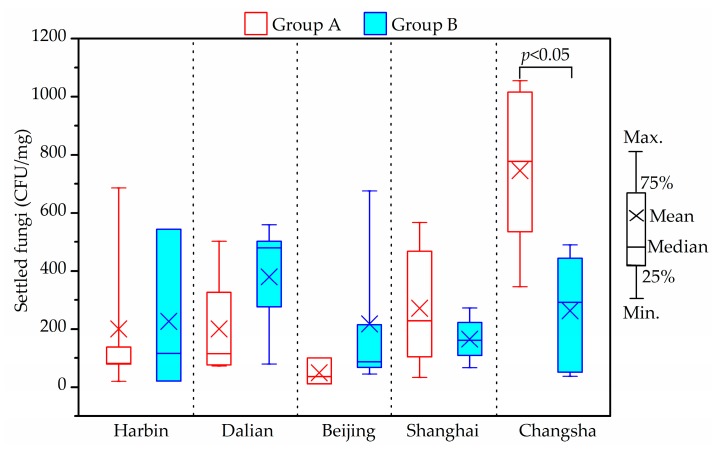
Figure 8.
Indoor average level of settled fungi in house dust in winter.
Figure 9.
Frequency of settled fungi species in house dust in winter.
The total bacterial count in all air samples was fond to be always below the upper limit of the 2500 CFU/m3 proposed by the Chinese national standards [56]. A stricter limit of 1000 CFU/m3 for airborne fungal spores in indoor air was suggested by the Architectural Institute of Japan [59]. Compared with this guideline, the indoor average airborne fungi level in one house in Group A from Harbin (1930 CFU/m3) and one house in Group B from Dalian (2048 CFU/m3) exceeded the limit. The airborne fungal levels in indoor air from Shanghai and Changsha samples were less than 500 CFU/m3. The maximum concentrations of indoor fungal found in Beijing was 845 CFU/m3, and detected in a house of Group B. However, there were no obvious differences between two groups (p > 0.05).
As shown in Figure 8 and Figure 9, yeast, Aspergillus, Wallemiasebi, Cladosporium and Penicillium were more frequently detected in house dust than other fungal species. The five dominant species accounted for around 92% of settled fungi in house dust. Some dust samples from Harbin, Dalian and Beijing were found to have no detectable fungi, due to the fewer samples that were taken.
The maximum indoor average settled fungi level was 1016 CFU/mg, and found in Changsha samples. The difference between Groups A and B for settled fungi from Changsha houses was significant (p < 0.05). However, no specific tendency, in this respect, was observed in the two groups in the other four cities (p > 0.05).
4. Discussion
4.1. Children’s Exposure to PM2.5, HCHO, Acetaldehyde and TVOCs
As regards this current study, human exposure pathways to air pollutants mainly include inhalation, oral ingestion and dermal absorption indoors [61]. Respiratory inhalation is the main route for PM2.5, HCHO, acetaldehyde and TVOCs. To evaluate children’s exposure to indoor environmental pollution, noting the time children spent in households was necessary if an accurate assessment record was to be achieved. According to the questionnaire responses in the six cities, the average daily time spent in living rooms and child’s bedroom was 4 h and 10 h, respectively. Personal inhalation exposure could be estimated by the methodology proposed by the United States Environmental Protection Agency (US EPA) [62]. The formula is described as Equation (1):
| (1) |
where Cj relates to the concentration of air pollutants measured in this study (μg/m3); IR relates to the inhalation rate of children (m3/h), in accordance with the recommended value by EPA [63]; t relates to the indoor exposure time (h), while j is the microenvironment room. Children’s daily exposures to pollutants, as indicated above, were given in detail in Table 4.
Table 4.
Child’s daily exposure to PM2.5, HCHO, acetaldehyde and TVOCs in the investigated households (μg/day).
| Item | Room | Dalian | Beijing | Shanghai | Wuhan | Changsha | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | ||
| PM2.5 | L | 239 | 96 | 107 | 727 | 392 | 368 | 1501 | 1324 | 491 | 687 |
| C | 699 | 194 | 340 | 1469 | 1249 | 1043 | 2490 | 3234 | 2831 | 2217 | |
| HCHO | L | 220 | 54 | 23 | 48 | 17 | 28 | 17 | 30 | 9 | 15 |
| C | 193 | 190 | 95 | 82 | 70 | 92 | 67 | 89 | 54 | 45 | |
| Acetal-dehyde | L | 52 | 39 | 13 | 52 | 9 | 12 | 19 | 23 | 10 | 16 |
| C | 83 | 97 | 55 | 50 | 38 | 36 | 57 | 69 | 62 | 47 | |
| TVOCs | L | 698 | 770 | 102 | 225 | 309 | 166 | 386 | 726 | 808 | 993 |
| C | 1411 | 1027 | 272 | 312 | 1170 | 2395 | 1112 | 1764 | 3570 | 2834 | |
| Total | 3594 | 2467 | 1008 | 2965 | 3255 | 4140 | 5649 | 7253 | 7835 | 6855 | |
L: living room; C: child’s bedroom; A, B: Groups A and B.
In most of the houses investigated, a child’s daily dose of air pollutants in the bedroom was significantly higher than that in the living room (p < 0.05), due to the longer time spent by the child in the bedroom over one day. The total daily dose of air pollutants which included PM2.5, HCHO, acetaldehyde and TVOCs in Groups A and B varied in different cities. In the Dalian and Changsha houses, the total daily doses of Group A, (namely Dalian: 3594 μg/day, Changsha: 7835 μg/day), were higher than those in Group B, (namely Dalian: 2467 μg/day, Changsha: 6855 μg/day). However, the opposite results were found in the Beijing, Shanghai and Wuhan houses.
4.2. Children’s Exposure to DBP and DEHP in House Dust
Children are exposed to DBP and DEHP from house dust through ingestion and dermal absorption [63,64,65]. Their daily intakes were estimated through Equations (2) and (3) as follows:
| (2) |
where is the DBP and DEHP level in house dust detected in the study (μg/g); is the percentage of time spent indoors over a day; is the dust ingestion rate, 0.05 g/day [64]; M is the body weight of children, as indicated in [66]:
| (3) |
where A is the body surface area (cm2/day). According to [64], A is 3067 cm2/day for children aged 9–10 years and 3692 cm2/day for children aged 11–12 years; m is the dust which adheres to skin, 0.096 mg/cm2 [64]; f3 is the fraction of DBP and DEHP absorbed in the skin, 0.001556 of DBP and 0.000106 of DEHP [64]. Table 5 presents a summary of the children’s daily doses of DBP and DEHP based on house dust present in different cities.
Table 5.
Child’s daily exposure to DBP and DEHP in the investigated households in winter (ng/day-kw/day).
| Room | Exposure Routes | Dalian | Beijing | Shanghai | Wuhan | Changsha | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | ||
| L | Ingestion | 448 | 580 | 211 | 347 | 478 | 708 | 92 | 318 | 110 | 167 |
| Dermal absorption | 1.5 | 1.7 | 0.6 | 2.0 | 1.9 | 2.3 | 0.5 | 1.6 | 0.3 | 0.6 | |
| C | Ingestion | 1866 | 2394 | 1178 | 1640 | 789 | 752 | 546 | 405 | 450 | 591 |
| Dermal absorption | 9.1 | 9.4 | 3.8 | 8.8 | 3 | 2.1 | 1.2 | 1.7 | 1.5 | 1.6 | |
| Total | 2325 | 2985 | 1393 | 1998 | 1272 | 1464 | 640 | 726 | 561 | 761 | |
L: living room; C: child’s bedroom; A, B: Groups A and B.
Children’s daily intake of DBP and DEHP from house dust in child’s bedrooms were significantly higher than such intakes from living rooms (Mann-Whitney U test, p < 0.05). However, the Pearson correlation coefficient (r) of daily exposure to DBP and DEHP betweenthe child’s bedroom and the living room showed that there was no significant correlations in the different rooms (r = 0.48, p > 0.05). The daily intakes of DBP and DEHP from house dust via ingestion varied from 638 to 2974 ng/day-kw/day, with an average of 1407 ng/day-kw/day, while 1.7 to 11.1 ng/day-kw/day with an average of 5.5 ng/day-kw/day through dermal absorption. Children in the houses from Dalian, Beijing and Shanghai ingested more phthalates from house dust than children from Wuhan and Changsha, with statistical significance (p < 0.05).
4.3. Relationships among Environmental Pollutants
In order to ascertain the possible relevance of measured environmental pollutants, Person correlation coefficients were calculated and are presented in Table 6. Concerning the correlations between pollutants in the living room (L) and the child’s bedroom (C), PM2.5, HCHO, acetaldehyde, settled fungi and DEHP presented strongly positive correlations (r > 0.85, p < 0.01), while TVOCs and DBP showed moderate correlations (r > 0.7, p < 0.05). It was quite clear that PM2.5 in particular, significant correlations were found between living rooms and child’s bedrooms, in five cities apart from Shanghai. In addition, indoor PM2.5 concentrations were significantly correlated with outdoors (r > 0.9, p < 0.01), thus suggesting that outdoor PM2.5 pollution has a strong influence on indoor PM2.5 pollution.
Table 6.
Pearson correlation coefficients for household environmental pollutants in six cities.
| Sites | Environmental Pollutants | Other Environmental Pollutants | Correlation Coefficient (r) | Sig. (2-Tailed) (p) |
|---|---|---|---|---|
| Harbin | PM2.5 (L) | PM2.5 (C) | 0.946 ** | 0.004 |
| Airborne fungi (L) | Airborne fungi (C) | 0.998 ** | 0.000 | |
| Dalian | PM2.5 (L) | PM2.5 (C) | 0.905 ** | 0.000 |
| Airborne fungi (L) | Airborne fungi (C) | 0.971 ** | 0.000 | |
| Settled fungi (L) | DBP (L) | 0.689 * | 0.04 | |
| Settled fungi (L) | DEHP (L) | 0.72 * | 0.029 | |
| Beijing [45] | PM2.5 (L) | PM2.5 (C) | 0.98 ** | 0.000 |
| PM2.5 (L) | PM2.5 (O) | 0.862 ** | 0.001 | |
| PM2.5 (C) | PM2.5 (O) | 0.874 ** | 0.001 | |
| HCHO (L) | Acetaldehyde (L) | 0.801 ** | 0.004 | |
| HCHO (C) | Acetaldehyde (C) | 0.775 ** | 0.007 | |
| Airborne fungi (L) | Airborne fungi (C) | 0.886 ** | 0.000 | |
| Airborne fungi (C) | PM2.5 (C) | 0.892 ** | 0.000 | |
| Settled fungi (L) | Settled fungi (C) | 0.974 ** | 0.000 | |
| Settled fungi (C) | DEHP (C) | 0.758 * | 0.042 | |
| Settled fungi (C) | DBP (C) | 0.805 * | 0.034 | |
| DEHP (L) | DEHP (C) | 0.873 ** | 0.000 | |
| Shanghai [46] | HCHO (L) | HCHO (C) | 0.833 ** | 0.003 |
| Acetaldehyde (L) | Acetaldehyde (C) | 0.904 ** | 0.000 | |
| Airborne fungi (L) | Airborne fungi (C) | 0.76 * | 0.011 | |
| Settled fungi (L) | PM2.5 (L) | 0.660 * | 0.038 | |
| Wuhan | PM2.5 (L) | PM2.5(C) | 0.965 ** | 0.002 |
| PM2.5 (L) | PM2.5 (O) | 0.927 ** | 0.008 | |
| PM2.5 (C) | PM2.5 (O) | 0.879 * | 0.021 | |
| HCHO (L) | HCHO (C) | 0.879 ** | 0.009 | |
| Acetaldehyde (L) | Acetaldehyde (C) | 0.946 ** | 0.001 | |
| HCHO (L) | Acetaldehyde (L) | 0.982 ** | 0.000 | |
| HCHO (C) | Acetaldehyde (C) | 0.96 ** | 0.001 | |
| DBP (L) | DBP (C) | 0.778 * | 0.039 | |
| Changsha [48] | PM2.5 (L) | PM2.5 (C) | 0.958 ** | 0.000 |
| PM2.5 (L) | PM2.5 (O) | 0.977 ** | 0.000 | |
| PM2.5 (C) | PM2.5 (O) | 0.921 ** | 0.000 | |
| TVOCs (L) | TVOCs (C) | 0.746 * | 0.013 |
L: living room; C: child’s bedroom; O: outdoor. * p < 0.05; ** p < 0.01.
From the above results, a correlation between living rooms and child’s bedrooms of airborne fungi was shown (p < 0.05). Meanwhile, airborne fungi were related to PM2.5 and settled fungi associated with DBP and DEHP. The correlations among different air pollutants were all statistically significant (p < 0.05). These relationships indicated that the associated compounds might come from the same indoor sources. Therefore, even if the levels of these mentioned pollutants did not exceed the upper limit, and the difference between the two groups of children’s exposures to these air pollutants was not significant, the combined influence of these pollutants could have adverse effects on the health of children. As a result, children’s health could be affected by the synthetic action of air pollutants such as PM2.5 and fungi.
4.4. Comparison of Environmental Conditions in Houses among Different Cities
This current study has examined indoor thermal and environmental conditions and their health risk to children in six Chinese cities. The environmental parameters in different cities displayed both similarities and differences. In terms of similarities, indoor levels of HCHO, acetaldehyde were practically lower than the guideline except for HCHO concentration in one investigated Dalian house as was acetaldehyde concentration in one measured Beijing house. Indoor average TVOCs levels were lower than 600 μg/m3 except in the selected Changsha houses.
The most frequently measured compounds in house dust in each city were DBP and DEHP. Lastly, the most frequently detected airborne fungi in the air were Cladosporium, Aspergillus and Penicillium. The differences between the different cities are given below:
-
(1)
As indoor heating equipment is generally used in winter in Northern cities such as Harbin, Dalian and Beijing, the indoor temperature in the houses varied in the range of 15–24 °C, and indoor average RH was less than 50%. Indoor RH in Beijing was relatively low, indoor RH in Shanghai and Changsha varied in the range of 60–75%. Whereas, the indoor RH percentage exceeding 70% in Changsha was approximately 33%, however, the rates of indoor RH exceeding 70% in Shanghai were more than 60%.
-
(2)
The CO2 levels in houses of Harbin, Dalian and Beijing were much higher than that in Shanghai, Wuhan and Changsha, as a consequence of poor natural ventilation caused by the use of indoor heating and low outdoor temperature.
-
(3)
The PM2.5 level in the investigated houses of Wuhan and Changsha were far higher than the upper limit. PM2.5 concentrations in other investigated cities were relatively low.
-
(4)
According to the Japanese standard, indoor airborne fungal levels in some investigated houses from Harbin and Dalian exceeded the limit of 1000 CFU/m3. The growth and reproduction of fungi flourish in a particular environment such as with a temperature range of 20–35 °C and a relative humidity range of 75–100% [67]. The indoor temperature in Harbin and Dalian were conducive to the growth and propagation of airborne fungi. Thus, the schoolchildren in Harbin and Dalian were naturally exposed to greater fungal pollution than those in Beijing, Shanghai and Changsha.
-
(5)
No obvious difference between settled fungi in Groups A and B in Harbin, Dalian, Beijing and Shanghai was found. In contrast, a significant difference was observed between the two groups in Changsha.
4.5. Limitations
There are some limitations in this study. The first potential limitation is the recall bias. The questionnaires were answered by children’s parents retrospectively. Generally, parents with family allergic history or whose child had been doctor-diagnosed with allergic diseases are likely to remember their children’s allergic symptoms and the related indoor environmental factors as well. However, some recall bias is inevitable. Secondly, in this study we only measured the pollutions of PM2.5, HCHO, TVOCs, SVOCs and fungi. It is important to further investigate the roles of the other air pollutants such as SO2 and NO2. Finally, the measurements did not take place over a full year, thus the indoor pollution conditions in other seasons is still unknown. Therefore, more detailed research work is needed in future.
5. Conclusions
A systematic study supported by a questionnaire survey and field investigation has been conducted. The focus areas were the following six typical cities in China: Harbin, Dalian, Beijing, Shanghai, Wuhan and Changsha. Field investigations focused on a selection of 10 houses in each city. Of prime interest was the children’s residential thermal environment and the identification of the extent and types of indoor primary pollutants in winter. Conclusions are drawn as follows:
-
(1)
Living environment factors such as homes near high traffic density roads, redecoration, small house size, dampness and lifestyles such as pets feeding within the family living space significantly contributed to allergic symptoms in children. Breast feeding (>6 months) was a protective factor for children suffering from pollen hypersensitivity and diagnosed allergies.
-
(2)
Indoor RHs in Group A (unhealthy children) of Dalian, and in many of those houses investigated in Groups A and B (healthy children) in Beijing were below 30%. The ratio of indoor RH exceeding 70% in Changsha and Shanghai were respectively about 35% and 60%. The above inappropriate thermal environments represent potential health risk factors as regards children’s health.
-
(3)
Much of the CO2 measured data exceeded Chinese limit level (1000 ppm) in different cities, possibly revealing that poor ventilation in houses could directly affect childhood health. The children in Group A in the investigated cities, except for Beijing were exposed to higher indoor PM2.5 levels than those in Group B according to Chinese national standard suggesting PM2.5 could be considered a risk factor for respiratory and allergic symptoms in children.
-
(4)
Except for one house in Dalian, HCHO concentration in all the other investigated houses did not exceed the upper limit of Chinese IAQ standard. Similar results were also observed for acetaldehyde levels, only one house in Beijing exceeded the guideline according to the Japanese national standard. Children in Group A in Changsha were exposed to higher TVOCs levels in their houses than those in Group B. Due to the synthetic action of these chemical compounds, it is reasonable to take them as important and prevalent indoor pollutants for the children, especially in child’s bedroom where children spent more time.
-
(5)
DBP and DEHP were the most frequently detected components of SVOCs in house dust. SVOC levels higher than 1000 μg/g (European Union standard) should be noted as potentially having adverse health effects.
-
(6)
Cladosporium, Aspergillus and Penicillium were simultaneously detected in indoor air and house dust, which could be suspected as adverse factors for children allergies.
These findings are helpful to evaluate and design healthy indoor living environment for schoolchildren, and provide some reference for future studies on assessing housing environmental risks for childhood health in China. Certainly, further research is needed to better understand the potential risk factors and the effect of indoor pollutants in other seasons of the year.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 51578220). The authors thank the residents who were involved in this study for their helpful cooperation. The authors also would like to thank warmly Hiroshi Yoshino, U. Yanagi and Shengwei Zhu for their valuable contribution in this study.
Author Contributions
Yang Lv, Jing Liu, Jingchao Xie, Huibo Zhang, Nianping Li and Jinhua Hu were main investigators for this project and contributed to study design and data collection. Jinhua Hu and Nianping Li analyzed the data; all authors interpreted the data; Jinhua Hu and Nianping Li wrote the manuscript with inputs from all authors. All authors have contributed to the study and approved the submission of the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Klepeis N.E., Nelson W.C., Ott W.R., Robinson J.P., Tsang A.M., Switzer P., Behar J.V., Hern S.C., Engelmann W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 2.Le C.P., Bonvallot N., Glorennec P., Deguen S., Goeury C., Le B.B. Indoor environment and children’s health: Recent developments in chemical, biological, physical and social aspects. Int. J. Hyg. Environ. Health. 2011;215:1–18. doi: 10.1016/j.ijheh.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Leech J.A., Nelson W.C., Burnett R.T., Aaron S., Raizenne M.E. It’s about time: A comparison of Canadian and American time-activity patterns. J. Expo. Anal. Environ. Epidemiol. 2002;12:427–432. doi: 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 4.Billionnet C., Gay E., Kirchner S., Leynaert B., Annesimaesano I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ. Res. 2011;111:425–434. doi: 10.1016/j.envres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Faustman E.M., Silbernagel S.M., Fenske R.A., Burbacher T.M., Ponce R.A. Mechanisms Underlying Children’s Susceptibility to Environmental Toxicants. Environ. Health Perspect. 2000;108(Suppl. 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai J., Zhao J., Shen K.L., Xiang L., Chen A.H., Huang S., Huang Y., Wang J.S., Ye R.W. Current Trends of the Prevalence of Childhood Asthma in Three Chinese Cities: A Multicenter Epidemiological Survey. Biomed. Environ. Sci. 2010;23:453–457. doi: 10.1016/S0895-3988(11)60007-X. [DOI] [PubMed] [Google Scholar]
- 7.Sundell J., Li B.Z., Zhang Y.P. China, Children, Homes, Health (CCHH) Sci. Bull. 2013;58:4179–4181. doi: 10.1007/s11434-013-9897-6. [DOI] [Google Scholar]
- 8.Chen Y.Z. Comparative analysis of the state of asthma prevalence in children from two nation-wide surveys in 1990 and 2000 year. Zhonghua Jiehe He Huxi Zazhi. 2004;27:112–116. [PubMed] [Google Scholar]
- 9.The National Cooperative Group on Childhood Asthma in China Third nationwide survey of childhood asthma in urban areas of China. Chin. J. Pediatr. 2013;51:729–735. [PubMed] [Google Scholar]
- 10.Brauer M., Hoek G., Smit H.A., de Jongste J.C., Gerritsen J., Postma D.S., Kerkhof M., Brunekreef B. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur. Respir. J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 11.Gasana J., Dillikar D., Mendy A., Forno E., Vieira E.R. Motor vehicle air pollution and asthma in children: A meta-analysis. Environ. Res. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Khreis H., Nieuwenhuijsen M.J. Traffic-Related Air Pollution and Childhood Asthma: Recent Advances and Remaining Gaps in the Exposure Assessment Methods. Int. J. Environ. Res. Public Health. 2017;14:312. doi: 10.3390/ijerph14030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y., Sundell J. Life style and home environment are associated with racial disparities of asthma and allergy in Northeast Texas children. Sci. Total Environ. 2011;409:4229–4234. doi: 10.1016/j.scitotenv.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Bornehag C.G., Sundell J., Hagerhed-Engman L., Sigsggard T., Janson S., Aberg N., DBH Study Group ‘Dampness’ at home and its association with airway, nose, and skin symptoms among 10,851 preschool children in Sweden: A cross-sectional study. Indoor Air. 2005;15:48–55. doi: 10.1111/j.1600-0668.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 15.Brunekreef B. Associations between questionnaire reports of home dampness and childhood respiratory symptoms. Sci. Total Environ. 1993;127:79–89. doi: 10.1016/0048-9697(92)90471-4. [DOI] [PubMed] [Google Scholar]
- 16.Brunekreef B., Dockery D.W., Speizer F.E., Ware J.H., Spengler J.D., Ferris B.G. Home dampness and respiratory morbidity in children. Am. Rev. Respir. Dis. 1989;140:1363. doi: 10.1164/ajrccm/140.5.1363. [DOI] [PubMed] [Google Scholar]
- 17.Nafstad P., Oie L.R., Gaarder P., Lodrup-Carlsen K., Botten G., Magnus P., Jaakkola J. Residential dampness problems and symptoms and signs of bronchial obstruction in young Norwegian children. Am. J. Respir. Crit. Care Med. 1998;157:410–414. doi: 10.1164/ajrccm.157.2.9706033. [DOI] [PubMed] [Google Scholar]
- 18.Tham K.W., Zuraimi M.S., Koh D., Chew F.T., Peng L.O. Associations between home dampness and presence of molds with asthma and allergic symptoms among young children in the tropics. Pediatr. Allergy Immunol. 2007;18:418–424. doi: 10.1111/j.1399-3038.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 19.Apte M.G., Fisk W.J., Daisey J.M. Associations between indoor CO2 concentrations and sick building syndrome symptoms in U.S. office buildings: An analysis of the 1994–1996 BASE study data. Indoor Air. 2000;10:246–257. doi: 10.1034/j.1600-0668.2000.010004246.x. [DOI] [PubMed] [Google Scholar]
- 20.Garrett M.H., Hooper M.A., Hooper B.M., Rayment P.R., Abramson M.J. Increased risk of allergy in children due to formaldehyde exposure in homes. Allergy. 1999;54:330–337. doi: 10.1034/j.1398-9995.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- 21.Rumchev K.B., Spickett J.T., Bulsara M.K., Phillips M.R., Stick S.M. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 2002;20:403–408. doi: 10.1183/09031936.02.00245002. [DOI] [PubMed] [Google Scholar]
- 22.Jaakkola J.J., Parise H., Kislitsin V., Lebedeva N.I., Spengler J.D. Asthma, wheezing, and allergies in Russian schoolchildren in relation to new surface materials in the home. Am. J. Public Health. 2004;94:560–562. doi: 10.2105/AJPH.94.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rive S., Hulin M., Baiz N., Hassani Y., Kigninlman H., Toloba Y., Caillaud D., Annesi-Maesano I. Urinary S-PMA related to indoor benzene and asthma in children. Inhal. Toxicol. 2013;25:373–382. doi: 10.3109/08958378.2013.790522. [DOI] [PubMed] [Google Scholar]
- 24.Neas L.M., Dockery D.W., Ware J.H., Spengler J.D., Ferris B.G., Speizer F.E. Concentration of Indoor Particulate Matter as a Determinant of Respiratory Health in Children. Am. J. Epidemiol. 1994;139:1088–1099. doi: 10.1093/oxfordjournals.aje.a116952. [DOI] [PubMed] [Google Scholar]
- 25.Nikasinovic L., Just J., Sahraoui F., Seta N., Grimfeld A., Momas I. Nasal inflammation and personal exposure to fine particles PM2.5 in asthmatic children. J. Allergy Clin. Immunol. 2006;117:1382–1388. doi: 10.1016/j.jaci.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Gaitanis G., Magiatis P., Hantschke M., Bassukas I.D., Velegraki A. The Malassezia Genus in Skin and Systemic Diseases. Clin. Microbiol. Rev. 2012;25:106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green B.J., Tovey E.R., Sercombe J.K., Blachere F.M., Beezhold D.H., Schmechel D. Airborne fungal fragments and allergenicity. Med. Mycol. 2006;44(Suppl. 1):S245–S255. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- 28.Kurup V.P., Shen H.D., Banerjee B. Respiratory fungal allergy. Microbes Infect. 2000;2:1101–1110. doi: 10.1016/S1286-4579(00)01264-8. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.L., Elfman L., Mi Y., Wieslander G., Smedje G., Norbäck D. Indoor molds, bacteria, microbial volatile organic compounds and plasticizers in schools: Associations with asthma and respiratory symptoms in pupils. Indoor Air. 2007;17:153–163. doi: 10.1111/j.1600-0668.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 30.Piecková E., Wilkins K. Airway toxicity of house dust and its fungal composition. Ann. Agric. Environ. Med. 2004;11:67–73. [PubMed] [Google Scholar]
- 31.Ozihel H. Cladosporium. Food Research & Action Center (FRAC) Press; Washington, DC, USA: 2011. [Google Scholar]
- 32.Pitt J.I., Basílico J.C., Abarca M.L., López C. Mycotoxins and toxigenic fungi. Med. Mycol. 2000;38(Suppl. 1):41. doi: 10.1080/mmy.38.s1.41.46. [DOI] [PubMed] [Google Scholar]
- 33.Yu A.B., Shibata E., Saito I., Araki A., Kanazawa A., Morimoto K., Nakayama K., Tanaka M., Takigawa T., Yoshimura T. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci. Total Environ. 2014;485–486:153–163. doi: 10.1016/j.scitotenv.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 34.Bornehag C.G., Sundell J., Weschler C.J., Sigsgaard T., Lundgren B., Hasselgren M., Hägerhedengman L. The Association between Asthma and Allergic Symptoms in Children and Phthalates in House Dust: A Nested Case-Control Study. Environ. Health Perspect. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolarik B., Naydenov K., Larsson M., Bornehag C.G., Sundell J. The Association between Phthalates in Dust and Allergic Diseases among Bulgarian Children. Environ. Health Perspect. 2008;116:98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu N.Y., Lee C.C., Wang J.Y., Li Y.C., Chang H.W., Chen C.Y., Bornehag C.G., Wu P.C., Sundell J., Su H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air. 2012;22:186–199. doi: 10.1111/j.1600-0668.2011.00753.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y.P., Li B.Z., Huang C., Yang X., Qian H., Deng Q.H., Zhao Z.H., Li A.G., Zhao J.N., Zhang X. Ten cities cross-sectional questionnaire survey of children asthma and other allergies in China. Sci. Bull. 2013;58:4182–4189. doi: 10.1007/s11434-013-5914-z. [DOI] [Google Scholar]
- 38.Deng Q., Lu C., Jiang W., Zhao J., Deng L., Xiang Y. Association of outdoor air pollution and indoor renovation with early childhood ear infection in China. Chemosphere. 2017;169:288–296. doi: 10.1016/j.chemosphere.2016.11.079. [DOI] [PubMed] [Google Scholar]
- 39.Deng Q., Lu C., Ou C., Chen L., Yuan H. Preconceptional, prenatal and postnatal exposure to outdoor and indoor environmental factors on allergic diseases/symptoms in preschool children. Chemosphere. 2016;152:459–467. doi: 10.1016/j.chemosphere.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z., Li A., Hu Z., Sun H. Study on the potential relationships between indoor culturable fungi, particle load and children respiratory health in Xi’an, China. Build. Environ. 2014;80:105–114. doi: 10.1016/j.buildenv.2014.05.029. [DOI] [Google Scholar]
- 41.Mi Y.H., Norbäck D., Tao J., Mi Y.L., Ferm M. Current asthma and respiratory symptoms among pupils in Shanghai, China: Influence of building ventilation, nitrogen dioxide, ozone, and formaldehyde in classrooms. Indoor Air. 2006;16:454–464. doi: 10.1111/j.1600-0668.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X., Zhao Z., Nordquist T., Larsson L., Sebastian A., Norback D. A longitudinal study of sick building syndrome among pupils in relation to microbial components in dust in schools in China. Sci. Total Environ. 2011;409:5253–5259. doi: 10.1016/j.scitotenv.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X., Zhao Z., Nordquist T., Norback D. The prevalence and incidence of sick building syndrome in Chinese pupils in relation to the school environment: A two-year follow-up study. Indoor Air. 2011;21:462–471. doi: 10.1111/j.1600-0668.2011.00726.x. [DOI] [PubMed] [Google Scholar]
- 44.Wei S., Lv Y., Fu B., Yoshino H. The Correlation Study on the Living Environment and Children’s Health Problem in Dalian. Procedia Eng. 2016;146:158–165. doi: 10.1016/j.proeng.2016.06.367. [DOI] [Google Scholar]
- 45.Fan G., Xie J., Yoshino H., Yanagi U., Hasegawa K., Kagi N., Liu J. Environmental conditions in homes with healthy and unhealthy schoolchildren in Beijing, China. Build. Environ. 2016;112:270–284. doi: 10.1016/j.buildenv.2016.11.045. [DOI] [Google Scholar]
- 46.Zhang H.B., Xie J.C., Yoshino H., Yanagi U., Hasegawa K., Kagi N., Lian Z.W. Thermal and environmental conditions in Shanghai households: Risk factors for childhood health. Build. Environ. 2016;104:35–46. doi: 10.1016/j.buildenv.2016.04.020. [DOI] [Google Scholar]
- 47.Zhu S.W., Cai W., Yoshino H., Yanagi U., Hasegawa K., Kagi N., Chen M.Q. Primary pollutants in schoolchildren’s homes in Wuhan, China. Build. Environ. 2015;93:41–53. doi: 10.1016/j.buildenv.2015.02.028. [DOI] [Google Scholar]
- 48.Hu J., Li N., Yoshino H., Yanagi U., Hasegawa K., Kagi N., He Y., Wei X. Field study on indoor health risk factors in households with schoolchildren in south-central China. Build. Environ. 2017;117:260–273. doi: 10.1016/j.buildenv.2017.03.014. [DOI] [Google Scholar]
- 49.GB 50176-93, Thermal Design Code for Civil Building. China Planning Press; Beijing, China: 1993. [Google Scholar]
- 50.Cui N., Xie J.C., Bian W.Y., Yoshino H., Yanagi U., Zhang H.B., Li Z.H., Liu J., Lv Y. Study on the Relationship between Indoor Air Quality and Children’s Health in Beijing. Appl. Mech. Mater. 2013;368–370:525–530. doi: 10.4028/www.scientific.net/AMM.368-370.525. [DOI] [Google Scholar]
- 51.Japan Standard Association . Indoor Air-Determination of Formaldehyde and Other Carbonyl Compounds-Active Sampling Method. Japan Standard Association; Tokyo, Japan: 2005. Japanese Industrial Standard (JIS) A 1962: 2005. [Google Scholar]
- 52.Japan Standard Association . Indoor Air-Sampling and Analysis of Volatile Organic Compounds by Sorbent Tube/Thermal Desorption/Capillary Gas Chromatography-Pumped Sampling. Japan Standard Association; Tokyo, Japan: 2005. JIS A 1966: 2005. [Google Scholar]
- 53.International Organization for Standardization . ISO 16000-18, Indoor Air-Part 18: Detection and Enumeration of Moulds Sampling by Impaction. International Organization for Standardization; Geneva, Switzerland: 2011. [Google Scholar]
- 54.International Organization for Standardization . ISO 16000-17. Idoor Air-Part 17: Detection and Enumeration of Moulds Culture-Based Method. International Organization for Standardzation; Geneva, Switzerland: 2008. [Google Scholar]
- 55.Yanagi U., Kagi N., Ikeda K. A study on the behavior and control of microbial contamination in an air conditioning system (Part 1) J. Environ. Eng. 2008;73:1197–1200. doi: 10.3130/aije.73.1197. [DOI] [Google Scholar]
- 56.GB/T 18883-2002, Indoor Air Quality Standard. Ministry of Environmental Protection of the People’s Republic of China; Beijing, China: 2002. [Google Scholar]
- 57.Woodfine L., Neal R.D., Bruce N., Edwards R.T., Linck P., Mullock L., Nelhans N., Pasterfield D., Russell D., Russell I. Enhancing ventilation in homes of children with asthma: Pragmatic randomised controlled trial. Br. J. Gen. Pract. 2011;61:724–732. doi: 10.3399/bjgp11X606636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.GB 3095-2012, Ambient Air Quality Standards. Ministry of Environmental Protection of the People’s Republic of China; Beijing, China: 2012. [Google Scholar]
- 59.AIJES-A0002-2013. Standards for Design and Maintenance of Indoor Air Pollution by Microbe. Architectural Institute of Japan; Tokyo, Japan: 2013. [Google Scholar]
- 60.European Comission Commission Delegated Directive 2015/863 of 31 March 2015 amending Annex II to Directive 2011/65/EU of the European Parliament and of the Council as regards the list of restricted substances. Off. J. Eur. Union. 2015:L137. [Google Scholar]
- 61.Agency for Toxic Substances and Disease Registry (ATSDR) Public Health Assessment Guidance Manual (Update) [(accessed on 25 April 2017)]; Available online: https://www.atsdr.cdc.gov/hac/phamanual/pdfs/phagm_final1-27-05.pdf.
- 62.United States Environmental Protection Agency Guidelines for Exposure Assessment. Fed. Regist. 1992;49:46304–46312. [Google Scholar]
- 63.United States Environmental Protection Agency Exposure Factors Handbook. [(accessed on 6 May 2017)];2011 Available online: https://www.nrc.gov/docs/ML1400/ML14007A666.pdf.
- 64.Guo Y., Kannan K. Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environ. Sci. Technol. 2011;45:3788–3794. doi: 10.1021/es2002106. [DOI] [PubMed] [Google Scholar]
- 65.Wang X., Tao W., Xu Y., Feng J., Wang F. Indoor phthalate concentration and exposure in residential and office buildings in Xi’an, China. Atmos. Environ. 2014;87:146–152. doi: 10.1016/j.atmosenv.2014.01.018. [DOI] [Google Scholar]
- 66.Report on National Physical Fitness Survellance (2010) General Administration of Sport of China; Beijing, China: 2011. [Google Scholar]
- 67.Zhong G.M., Chen L.X. Progress of the study on indoor microbial contamination and anti-microorganism technique. J. Environ. Health. 2005;22:69–71. [Google Scholar]