Abstract
Rotator cuff pathology is a significant cause of shoulder pain. Operative repair of rotator cuff is an established standard of care for these patient, however, failure of the procedure is common. Systemic conditions such as diabetes mellitus, hypocholesteremia, thyroid disease, and smoking significantly affect the outcomes of rotator cuff repair and have significant implications for the management of these patients. Diabetes mellitus has been proposed to damage tendons through non-enzymatic glycosylation of collagen with advanced glycation end product formation and impaired microcirculation. Hypocholesteremia may lead to fatty infiltration and subsequent pro-inflammatory degenerative enzymatic degeneration. Thyroid disease may disrupt tendon homeostasis through the alteration of collagen production and the accumulation of glycosaminoglycans. Lastly, smoking inhibits tendon healing through the induction of hypovascularity and hypoperfusion. Understanding of the implications these systemic conditions have on the outcomes is important in the management of rotator cuff disease.
Introduction
Rotator cuff pathology is a significant cause of shoulder pain, with over 50% of the general population affected with partial to full thickness tears by the age of 60 years.1 Operative repair of rotator cuff is an established standard of care for these patient, however, failure of the procedure is common and well documented.1 Rotator cuff disease is due to a complicated spectrum of pathology and sub-optimal outcomes of both treatment may stem from inadequate management of the contributory causes. The proposed mechanisms of rotator cuff tendinopathies and injuries can be generally grouped into intrinsic and extrinsic factors. Pathologic alterations originating from the cuff tendon itself comprise the the intrinsic factors and include degeneration, repetitive micro trauma, and vascular compromise.1 Extrinsic factors include the shape of the acromion, glenohumeral impingement and dislocation, overuse syndromes, and several demographic factors.1,2 Systemic conditions such as diabetes mellitus, hypocholesteremia, thyroid disease, and smoking significantly affect the outcomes of rotator cuff repair and have significant implications for the management of these patients.
Diabetes Mellitus
Diabetes mellitus is one of the most systemically debilitating diseases in the world, and profoundly affects musculoskeletal tissue function and regeneration following injury.3,4 The prevalence of this disease is estimated to affect approximately 17.5 million people in the United States and is expected to continue expanding in an accelerated rate.4 A higher prevalence of shoulder disorders (27.5%) has been demonstrated in patients with diabetes as compared to the rate found in other general medical patients (5.0%), and this is expected to continue to grow in parallel with the increasing rate of disease in the population.5 Several epidemiologic studies have associated shoulder disorders and rotator cuff pathology with diabetes. In a cross-sectional population study of 6237 Finnish patients, Rechardt et al. showed that both types 1 and 2 diabetes mellitus were associated with a higher prevalence of shoulder pain and rotator cuff tendinitis in men.6 In a recent large-scale population-based study, Lin et al. found that patients with diabetes had a 2.11-fold increased risk of rotator cuff disorders in the Taiwanese population.7 Similarly, Ranger et al. performed a recent systematic review with meta-analysis and concluded that rotator cuff tendinopathy and thicker rotator cuff tendons were significantly more prevalent in diabetic patients.8
A frequency matched case-control study by Longo et al. demonstrated that patients with rotator cuff tears had significantly higher fasting plasma glucose levels as compared to a control population.9 Interestingly, these elevated levels still resided within the normal glycemic range, leading the authors to conclude that even increased plasma glucose in subclinical diabetes was associated with rotator cuff tears. Utilizing ultrasound, Akturk et al. demonstrated significantly thicker biceps and supraspinatus tendons (RR: 22.75 and 24.28 respectively) in a diabetic cohort as compared to a control population, showing clear anatomic abnormalities associated with the disease.10 The diabetic phenotype is associated with decreased collagen content and cross-linking defects,11–16 a propensity for shoulder stiffness,17–22 and increased risk of both complications and infections following open rotator cuff repair23–25 in both clinical and basic science literature.
Effect on Tendon Healing & Biomechanics
The exact mechanism by which diabetes contributes to rotator cuff pathology has not been specifically identified. However, it is postulated that non-enzymatic glycosylation and impaired microcirculation at the rotator cuff tendons could be significant contributory factors. Non-enzymatic glycosylation of collagen with advanced glycation end product (AGEs) formation has been proposed to be a major pathway for tendon degeneration and inflammation.26 AGEs accumulate after rotator cuff tear, and likely alter the rotator cuff tendon biochemical and mechanical properties through increased intermolecular collagen cross-linking.27 The process in which collagen cross-links alter global tendon function is complex and continues to be an area of developing study. However, it is generally accepted that AGE collagen cross-links results in increased stiffness and brittleness of collagen tissues, leading to worsening mechanical fragility.28,29 Inhibition of collagenous tissue remodeling and the reduction of viscoelastic properties in tendon structures leading to an impairment of fiber-to- fiber sliding due to AGE collagen cross-links are some proposed mechanical pathways leading to rotator cuff tendinopathy and injury.28,30
AGEs may also upregulate local inflammatory responses at the rotator cuff tendon. Valencia et al. found specific receptors for AGEs on chondrocyte and tenocyte cell membranes.31 The binding of this receptor has been shown to enhance the transcription of nuclear factor NF-κB and the upregulation of proinflammatory mediators which produces cell cycle arrest.32 Additionally, diabetic patients have demonstrated increased expression of pro-inflammatory cytokines and vascular endothelial growth factors.33 These inflammatory reactions within the rotator cuff tendon of diabetics may therefore lead to a higher risk of tendon pathology and injury. The culmination of these biochemical alterations leads to biomechanical derangement as Boivin et al. found that diabetic mice demonstrated tendons with decreased values in maximum load, tensile stress, stiffness and elastic modulus.34 While diabetes has been associated with thicker tendons, the biomechanical properties of these affected tissues have actually been shown to be of inferior quality, collagen fibers becoming smaller, denser, and stiffer.35
Repeated microtrauma secondary to glycation-induced changes in the mechanical properties of tissue may also compound the damage experienced by the rotator cuff tendons of diabetic individuals. Chbinou and Frenette found alterations of angiogenic, inflammatory, and proliferative processes involved in tendon healing and remodeling with the induction of repeated trauma in a diabetic tendinitis model.36 Handa et al. demonstrated a substantial elevation in the expression of vascular endothelial growth factor and altered angiogenesis in patients with type II diabetes presenting with shoulder contractures and rotator cuff disease as compared to controls.33 Additionally, Bedi et al. demonstrated in a rat model that sustained hyperglycemia impairs rotator cuff tendon healing at the enthesis. Interestingly, this study showed that hypoglycemia also led to impaired tendon healing and increased stiffness in euglycemic rats.37 Diabetic animals in this study produced significantly less fibrocartilage and organized collagen at the level of the healing enthesis. Significantly impaired ultimate load to failure and stiffness was also found at the healing enthesis. Immunohistochemistry demonstrated significantly increased deposition of AGEs in the diabetic animal, while they were virtually absent in control animals.
Clinical Outcomes
Poor structural outcomes following rotator cuff tendon repair have been reported in diabetic patients. Zakaria et al. demonstrated that the risk of rotator cuff tendon rupture requiring hospitalization in an Australian diabetic population was increased (OR: 1.84) as compared to the general population.38 Cho et al. performed a comparative cohort study and demonstrated that patients with diabetes had an significantly increased retear rate (35.9%) following arthroscopic rotator cuff repair surgery as compared to controls (14.4%) as evaluated on MRI (P < .001).39 These authors also found that effective glycemic control was associated with improved healing rates after rotator cuff repair, with 43.2% in an uncontrolled diabetic patient population (>7.0% hemoglobin A1c) as compared to 25.9% in patients with adequate glycemic control developing re-tears of the repaired tendon.
Increased shoulder stiffness is also a significantly reported adverse effect among diabetic patients following rotator cuff repair. Blonna et al. found that subclinical diabetes discovered at the time of surgery was a risk factor for moderate stiffness postoperatively (risk ratio = 5.7) in a prospective study of 65 consecutive patients undergoing rotator cuff repair.40 In patients who were previously diagnosed with diabetes or showed prediabetes conditions, 42% went on to develop postoperative stiffness. Chen et al. also showed in a comparative study that diabetic patients had decreased shoulder range of motion, a higher rate of failure, and an increased postoperative infection rate of 10% as compared to non diabetic patients.41 Importantly, however, these authors utilized a mini open technique which has previously been associated with increased infection rates as compared to arthroscopic rotator cuff repair.42 WhileChen et al. concluded that diabetic patients can expect functional improvement after surgery, they did note significant decreases in shoulder active and passive range of motion at six weeks, six months, and 34 months after rotator cuff repair as compared to non diabetic patients. Conversely, while other clinical studies have also demonstrated inhibited shoulder range of motion in the short-term postoperatively in diabetic patients, long-term follow-up do not demonstrate significant differences between these patients and non diabetic controls.43,44
Few studies have described comparative results in patient reported functional outcomes scores among diabetic patients following rotator cuff repair, but the current literature suggests that the disease produces suboptimal outcomes. Clement et al. demonstrated inferior improvements in the Constant-Murley score at 6 months (18.8 vs. 11.6,) in a diabetic cohort following arthroscopic rotator cuff repair as compared to an age, sex, tear size, and comorbidity matched cohort.24 Similarly Chung et al. showed that diabetes has a significant effect on postoperative SF-36 and shoulder-specific functional outcome scores.
Effect of Modifications
There have been various proposed approaches to prevent formation of AGEs in addition to recent research directed towards therapeutic options aiming to undo collagen cross-linking. However, most AGEs cross-link breaking therapies have been directed towards other tissues such as dermis and vascular organs. The goals of these treatments would aim to potentially reverse the damaging effects on tendon tissue biomechanics and remodeling. One of the first studies to demonstrate the potential effectiveness of these therapeutics on tendon tissues was shown by Vasan et al., who showed that advanced glycosylation and product cross-links are susceptible to thiazolium compounds in a rat tail tendon model.45 These compounds selectively cleaves AGE crosslinks in rat-tail tendon both in-vitro and in-vivo, demonstrating a decrease in AGE crosslinks after 32 weeks of treatment.45 Since that time, several other compounds have been analyzed to determine their efficacy in breaking AGE cross-links, however, these potential therapeutics remain in the experimental phase.
Sustained hyperglycemia and diabetes mellitus has been demonstrated to significantly impair tendon to bone healing and produce inferior outcomes for patients after rotator cuff repair. The presence of this medical comorbidity in the setting of proposed surgery represents important implications for preoperative evaluation and counseling for patients with impaired glycemic control in regards to expectations postoperatively. The management of rotator cuff tendinopathy and other orthopedic pathology in a diabetic patient may be benefited from a multidisciplinary perioperative evaluation to improve morbidity and functional outcomes while minimizing postoperative complications in this at risk population.46
Hypercholesterolemia
Hypercholesterolemia and hyperlipidemia have been suggested as important extrinsic factors contributing to rotator cuff pathology. Fatty infiltration has been demonstrated to be an important prognostic factor in the functional and structural outcomes following rotator cuff repair.47 Cholesterol is known to result in the accumulation of fatty deposits in muscle and tendon tissues as demonstrated with familial hypercholesterolemia, and it is postulated that this process may positively augment the fatty infiltration pathology of rotator cuff tendinopathy and accelerate the disease secondary to structural derangement of the collagen matrix.48,49 Previous studies have demonstrated that hypercholesterolemia results in fatty deposition in several tissues including muscle and tendons. 50,51 This fatty deposition can interfere with proper function of the contractile and mechanical proteins in tissue, and animal models have indicated that the use of cholesterol lowering drugs such as simvastatin can improve functional measures of recovery after rotator cuff tear.52,53 Lin et al. showed that hyperlipidemia was an independent risk factor in the development of rotator cuff disease in the Taiwanese population.7
Abboud and Kim prospectively collected serum lipid profiles on a cohort of 74 patients with rotator cuff tendon rupture's in comparison to a 73 patient age matched control group with non-tendon related shoulder complaints. The authors found that hypercholesterolemia was more prevalent in the rotator cuff court (63%) as compared to the control group (28%).54 However, another study by Longo et al. demonstrated no association among rotator cuff tendinopathy and hypercholesterolemia in a comparison of 120 arthroscopic rotator cuff repairs as compared to a group of 120 arthroscopic meniscectomies.9
The ability to successfully repair the torn cuff and promote the return of patients to normal strength and function is often complicated by fibrosis, atrophy and fatty infiltration of the rotator cuff muscle.55 The extent of fatty infiltration within rotator cuff muscles have therefore been shown to increase the risk of structural failure after surgical repair, as well as poor functional outcomes.56 However, despite operative intervention, fatty infiltration has been shown to continue after surgical repair and correlates with continued poor functional outcomes.47 Due to an increasingly aging population and decreasing preventative measures such as healthy dietary habits and exercise, the prevalence of hypercholesterolemia is quickly increasing.
Effect on Tendon Healing & Biomechanics
High serum cholesterol levels lead to an elevation of oxidized low density lipoproteins (LDLs), which have been shown to produce local pro-inflammatory effects, leading to a more susceptible environment favoring cell apoptosis. Beason et al. has shown that cholesterol deposition within tendons produce chronic degeneration and persistent inflammation of the rotator cuff.57 Fatty deposition secondary to hypercholesterolemia increases cytokines such as interleukin-6 and tumor necrosis factor alpha, subsequently initiating and maintaining proinflammatory inhibition of tendon to bone healing.58 Deranged micro- and macro-circulation at the critical zone of the rotator cuff tendon enthesis secondary to hypercholesterolemia may also result in predisposition to injury and delay optimal healing.9 These combined effects may potentiate each other for an unfavorable healing environment for the rotator cuff.
Hypercholesteremia has also been shown to cause to formation of xanthomas, accumulations of lipid laden macrophages within tendon tissues, often seen in patients with familial dyslipidemias. These accumulations are typically formed originating from circulating plasma lipids as opposed to local synthesis.59 Xanthoma accumulation in tendon tissues may lead to structural disruption as well as local inflammation, resulting in tendinopathy. Jozsa et al. demonstrated this as lipid deposits along collagen fibrils which were observed utilizing electron microscopy, possibly disrupting the structural integrity of the tendon and making it more prone to tendon rupture or injury.60
Fatty accumulation in existing adipocytes, adipocyte differentiation, and adipocyte differentiation from pluripotant cells have been various proposed mechanisms of progressive rotator cuff fatty infiltration in the setting of hypercholesterolemia.61 Killian et al. demonstrated that tendon unloading following rotator cuff rupture produced an upregulation of pro-adipogenic genes including CCAAT/enhancer binding protein-α (c/ebpα) and peroxisome proliferator-activated receptor-γ (pparγ), and leptin.62 Chung et al. utilized a chronic supraspinatus tear rabbit model and demonstrated that animals who were fed a high cholesterol diet showed significantly decreased compound muscle action potentials on EMG, lower mechanical properties in both load to failure and stiffness, and a significantly higher fat to muscle proportion.63 Importantly, high cholesterol diet animals treated with simvastatin demonstrated a decreased fat to muscle proportion as compared to high cholesterol diet animals without simvastatin.63 Control animals and high cholesterol animals receiving simvastatin also had better tendon to bone interface structures with improved collagen organization as compared to high cholesterol diet animals.63 Oh et al. utilized a chronic rabbit subscapularis tear model and demonstrated that the addition of adipose-derived stem cells decreased the proportion of fat infiltration histologically with the resultant larger compound muscle action potential as compared to control repairs which demonstrated a greater proportion of fat infiltration, reflecting the findings of Chung et al.64
Hypercholesterolemia appears to negatively alter the biomechanical properties of rotator cuff tendons as well. The accumulation of fibrotic tissue is thought to impair the healing potential and elasticity of a rotator cuff repair.65 Beason et al. used a rotator cuff tendon repair rat model to evaluate tendon mechanical properties following exposure to a high cholesterol diet over the course of six months in an attempt to evaluate any alterations in the time course of supraspinatus healing.57 Biomechanical testing demonstrated a significant reduction in normalized stiffness in animals exposed to the hypercholesterolemic diet at four weeks. However, no significant differences were detected at eight weeks and histological analysis did not demonstrate any significant differences in collagen organization, cellularity, or so shape compared to controls. Beason et al. then demonstrated that increased supraspinatus tendon stiffness and elastic modulus was present in the setting of hypercholesterolemia across multiple species.66
There is a paucity of clinical outcome studies directly comparing rotator cuff tendinopathy patients with and without hypercholesterolemia. Kim et al. compared 50 non-hyperlipidemia with 49 hyperlipidemia patients presenting with supraspinatus tendinopathy (with and without tears).67 The authors found that pain improvement was inferior in the hyperlipidemia group as compared to the non-hyperlipidemia group by a numeric rating scale. They also found that passive range of motion in the non-hyperlipidemia group was increased, however, this finding was not statistically significant. Importantly, however, the prevalence of diabetes mellitus in the hyperlipidemia group (28.9%) was higher than in the non-hyperlipidemia group (6.38%).
Statin Therapy
Statins are drugs which lower serum cholesterol levels by inhibiting the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is involved in cholesterol production in the liver.68 Previous studies involving simvastatin in relation to the Achilles tendon have demonstrated potentially increased risk of rupture.69 However, more recent literature has produced some interesting insights in the role of statins as a protective and therapeutic agent in the setting of rotator cuff pathology.
Lin et al. found that in a subgroup of patients with hyperlipidemia, the use of statins were associated with a lower risk of developing rotator cuff disease (rosuvastatin: HR, 0.41 [95% CI, 0.35-0.49] ; P < .0001) (simvastatin: HR, 0.62 [95% CI, 0.54-0.71] ; P < .0001) (other statins: HR, 0.66 [95% CI, 0.60-0.72] ; P < .0001), suggesting that statin usage may provide some protection against pathology in patients with hyperlipidemia.7 Davis et al. used an animal model to show that Simvastatin partially protected muscles from weakness secondary to chronic rotator cuff tears.53 Fibrosis was also markedly reduced in these simvastatin-treated animals.53
Tucker and Solslowsky found that rats with hypercholesteremic diets treated with simvastatin showed increased tendon cross-sectional area and decreased modulus. Additionally, the simvastatin group had a higher observable proportion of spindle shaped cells.70 However, the authors conclude that it is unclear whether these structural changes would provide any biomechanical benefit. Dolkart et al. used a rat model to show that treatment with atorvastatin in a rotator cuff repair model resulted in significantly higher maximum load to failure. These authors also demonstrated that atorvastatin treated tenocytes demonstrates significantly higher proliferation rates and induced migration and adhesion of these cells. They concluded that atorvastatin enhances tendon healing by stimulating tenocyte proliferation, migration, and adhesion via increased Cyclooxygenase activity and autocrine/paracrine prostaglandin E2signaling.71
Thyroid Disease
It is not clear to what level a patient's hormone homeostasis contributes to the development of rotator cuff tendinopathy and what the effect it has on tendon healing. However, recent literature has suggested an important role of thyroid hormones in the development of shoulder tendinopathies.72,73 Thyroid hormones play a critical role in the regulation of a multitude of adult metabolic functions from gene regulation to influencing thermogenesis, cellular growth, and affecting mitochondrial processes.74 Accumulation of glycosaminoglycans (GAGs) in the extracellular matrix because of hypothyroidism may predispose tendons to pathology. Often, tendinopathy may be the presenting symptoms in a patient with hypothyroidism, with often successful relief after management of their endocrine disorder.75 Calcified tendinopathy has also been associated with thyroid dysfunction.76 A case report of spontaneous rupture of the long head of the biceps tendon in a woman with hypothyroidism has been presented in the literature.77
Effect on Tendon Healing & Biomechanics
Triiodothyronine (T3) is the main mediator of metabolic effect in relation to thyroid hormones. T3 binds to thyroid hormone receptors and regulates gene expression via the (TR)-α and –β cell surface receptors, binding enhancer elements at target genes and producing both positive and negative regulatory actions on the transcription of genes.78 The relationship between thyroid hormones and musculoskeletal complaints, particularly shoulder issues have been described for decades, with recent theories associating thyroid pathology with tendonopathies.72,73,79 Both (TR)-α and –β cell surface receptors have been detected on tenocyte's, indicating the critical role of thyroid hormones in regulating tenocyte cell proliferation and the potential for tendinopathy in the setting of endocrine disorders such as thyroid disease.
Olicia et al. demonstrated that (TR)-α and –β nuclear receptor isoforms are express at high levels on both native and pathologic rotator cuff tendons. Thyroid hormone induced tenocyte proliferation in both healthy and pathologic tenocytes. Interestingly, the authors did not detect a difference in the levels of receptor expression between healthy tenocytes and tenocytes derived from tendons obtained from patients with thyroid disease. Taken together, these results reinforce the concept of a physiological action of thyroid hormones in the homeostasis of tendons as an extrinsic factor.80
Thyroid hormone have been suggested to be a central player in the synthesis of collagen and the metabolism of extracellular matrix.81,82 Thyroxine (T4) has been demonstrated to be important in collagen synthesis and the formation of extracellular matrix metabolism. The accumulation of GAGs have been linked to hypothyroidism and is a proposed contributor to the development of calcification of tendons.83 Production of extracellular matrix proteins in vitro by tenocyte's have also been demonstrated to be influenced by thyroid hormones. Cultivation of tenocyte's with T3 or T4 individually or in combination with ascorbic acid was shown to increase the expression of collagen Iin the extracellular matrix. Cartilage oligomeric matrix protein (COMP) expression was also found to be upregulated. COMP is a modulator of collagen fibrils, and is able to bind to collagen type I, II, and IX, as well as fibronectin.84 Tenocytes are a specialized subset of fibroblasts, contributing to ECM homeostasis. T3 and T4 has been suggested to be anti-apoptotic mediators on tenocytes by Oliva et al.80 Thyroid hormones are thus suggested to significantly contribute to the general homeostasis of the tendon environment and disruptions in this mechanistic pathway could predispose tissues to injury.
Clinical Outcomes
In a retrospective observational cohort of 441 patients undergoing rotator cuff repair, Olivia et al. showed an association of thyroid hormones with a higher rate of non-traumatic rotator cuff tears. Thyroid disease was indirectly associated with cuff tears as the authors noted that females in this cohort showed a higher frequency (63%) of thyroid pathology.85 Harvie et al. performed an observational cohort study of 102 patients with calcific tendinitis and demonstrated that the prevalence of hypothyroidism in this pathologic population was significantly higher as compared to normal population estimates.76 Blonna et al. analyzed 65 consecutive patients scheduled for arthroscopic subacromial decompression or rotator cuff tear repair and found that patients presenting with hypothyroidism were at risk of developing severe postoperative stiffness (risk ratio = 25), with 75% of these patients developing postoperative stiffness.40 While there is a lack of clinical outcome literature associating thyroid pathology with rotator cuff tendinopathy, there is a growing recognition of the prevalence of autoimmune thyroid diseases in patients with connective tissue disorder, potentially indicating a common mechanism for this disease pathogenesis.86,87
Smoking
Smoking has clearly been demonstrated to have a deleterious effect on the musculoskeletal system.88,89 The prevalence of smoking remains close to 20% in the general population, being highest in persons below the poverty level or aged 22 to 44 years. Jeong et al. found that the prevalence of full-thickness rotator cuff tears was higher in patients with a smoking history (P=.002). Baumgarten et al. found that a history of smoking (61.9% versus 48.3%), smoking within the last 10 years (35.2% versus 30.1%), mean duration of smoking (23.4 versus 20.2 years), mean packs per day of smoking (1.25 versus 1.10 packs per day), and mean pack-years of smoking (30.1 versus 22.0) was correlated with an increased risk for rotator cuff tear.90 This relationship was found to be dose and time dependent.
Smoking impairs tissue oxygenation, alters the native inflammatory and restorative functions of affected cells, and leads to delays in tissue healing.91 The effects of smoking are thought to imbalance the delicate microvascular environment of the relatively hypovascular rotator cuff tendons and to negatively affect cuff pathology.92 Vascular insufficiency to the critical portion of the supraspinatus/infraspinatus tendon has a well defined contribution to the genesis of rotator cuff tear.93 This limitation on healing potential results in poor functional outcomes following surgical interventions.94
Effect on Tendon Healing & Biomechanics
Galatz et al. demonstrated that nicotine negatively affected healing, showing persistent inflammation and impaired cellular proliferation, impairing tendon-to-bone healing in a rotator cuff repair rat model.95 The authors also found that nicotine also resulted in decreased collagen type I expression and biomechanical properties were also generally inferior in the treated cohort as compared to controls. The authors proposed that these effects culminate in impaired scar maturity and remodeling at the enthesis site, potentially secondary to a negative effect on ECM degradation. Jorgensen et al7 analyzed the effects of smoking on collagen deposition in wound healing and found that smokers had 1.8 times less mature collagen in their surgical wounds. The amount of collagen deposition and repair at the wound was negatively correlated with the consumption of tobacco.96 Ichinose et al. has also demonstrated alterations in biomechanical properties of the supraspinatus tendon in the setting of nicotine, producing increased elastic modulus of the supraspinatus tendon in a rat model, an indicator for increased stiffness which is more predisposed to injury.97 Nicotine has been reported to elevate collagen synthesis and increased collagen cross-linking in animal models. These processes may lead to increased tissue fibrosis and subsequent higher elastic modulus.97
Macro- and microcirculation insufficiency to the critical portions of the rotator cuff are implicated in the development of tendinopathies and tears.93 Several studies provide strong evidence of the relative hypovascularity of both the supraspinatus and infraspinatus tendons in the vicinity of their insertion onto the greater tuberosity.93,98,99 It has been demonstrated that the area of the supraspinatus and infraspinatus tendons within 15 mm of their insertion into the greater tuberosity is relatively hypovascular.92 Nicotine induced hypoxia for the susceptible cuff tendon regions inhibit native metabolism and impair appropriate healing. Daftari et al. have demonstrated that nicotine inhibits the revascularization of bone graft in spinal fusions.100 Lundgreen et al. found that supraspinatus tendon samples from smokers with full-thickness tears demonstrated increased density of apoptotic cells accompanied by reduced tenocyte density and upregulation of proliferative activity.101 Given that the establishment of a vascular supply to the rotator cuff tendon is one of the critical factors in effective cuff healing, smoking as a pathologic mechanism for impaired healing is significantly supported.
Clinical Outcomes
Inferior structural outcomes of rotator cuff repair have also been demonstrated in association with smoking. Neyton et al. demonstrates significantly lower rate of healing compared with nonsmokers.102 Fourteen (78%) patients among the smokers had a healed tendon compared with 79 (93%) of the nonsmokers, demonstrating impairment of tendon-to-bone healing as observed on MRI. Evaluation of tendon healing with ultrasound have yielded more inconsistent results. Nho et al. found no association with smoking and cuff tendon defects on ultrasound.103 Tashjian et al. also failed to find an association with smoking and tendon failure via ultrasound, interestingly, these authors found that smoking status was actually a protective factor for rotator cuff failure (OR: 5.50).104 However, if is important to note that this finding is resultant from a very small sample size of smokers within their cohort. Carbone et al. compared 131 smokers who underwent arthroscoptic rotator cuff repair and showed that smokers were significantly more associated with having larger full thickness tears.105 They also found that increasing number of daily cigarettes reported were associated with increasing severity of tear size.
Balyk et al. showed that patients who were Worker's Compensation Board recipients were more likely to be smokers and had inferior Western Ontario Rotator Cuff Index (WORC) scores at 6 months postoperatively.106 Mallon et al. retrospectively compared 95 smokers and 129 non-smokers undergoing rotator cuff repair and found significantly higher mean improvement in total UCLA score (14.1 ± 5.8 vs. 9.1 ± 6.9,) and higher improvement in subjective assessment of pain (5.3 ± 2.1 vs. 3.4 ± 2.6) in non-smokers. Smokers also demonstrated higher pre-operative pain scores and lower preoperative UCLA scores. Almeida et al. demonstrated that there was a significantly higher UCLA score amount non-smokers as compared to smokers with large and extended rotator cuff tears which were arthroscopically repaired.107 However, These findings are not ubiquitous as Prasad et al. did not find a significant effect of smoking status on post-operative Constant-Murley scores or VAS pain scores.108
Discussion
Diabetes mellitus, hypocholesteremia, thyroid disease, and smoking significantly impairs the biologic, biomechanical, and functional outcomes of rotator cuff repair (Figure 1). Additional research is required to further delineate the specific mechanisms by which each of these conditions affect rotator cuff tendon repair, possibly allowing the development of future therapeutic and preventative measures to improve outcomes. It is important for surgeons to understand the implications these prognostic factors secondary to systemic conditions have on the outcomes and provide appropriate expectations and counseling.
Figure 1.
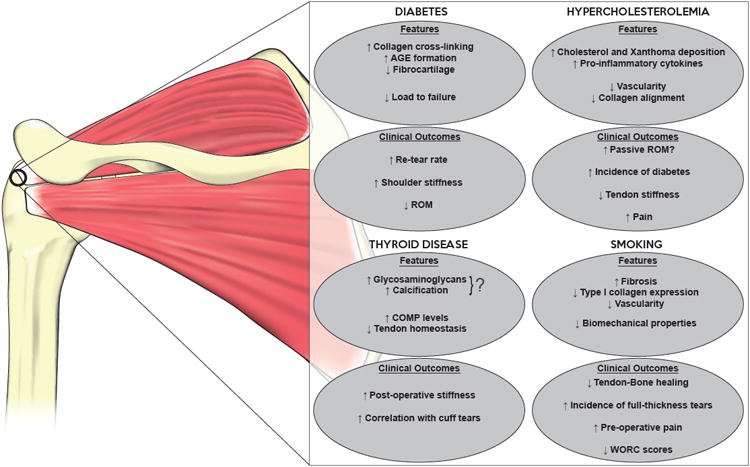
Overview of effects of systemic conditions on biological properties and clinical outcomes of the rotator cuff following repair. (AGE: advanced glycation end-product; ROM: range of motion; COMP: cartilage oligomeric matrix protein; WORC: Western Ontario Rotator Cuff Index)
Contributor Information
Simon Lee, University of Michigan.
Jonathan Gumucio, University of Michigan.
Christopher Mendias, University of Michigan.
Asheesh Bedi, University of Michigan.
References
- 1.Nho SJ, Yadav H, Shindle MK, Macgillivray JD. Rotator cuff degeneration: etiology and pathogenesis. Am J Sports Med. 2008;36(5):987–993. doi: 10.1177/0363546508317344. [DOI] [PubMed] [Google Scholar]
- 2.Titchener AG, White JJE, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg. 2014;23(9):1282–1288. doi: 10.1016/j.jse.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Smith LL. Musculoskeletal manifestations of diabetes mellitus. British Journal of Sports Medicine. 2003;37(1):30–35. doi: 10.1136/bjsm.37.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 5.Thomas SJ, McDougall C, Brown IDM, et al. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J Shoulder Elbow Surg. 2007;16(6):748–751. doi: 10.1016/j.jse.2007.02.133. [DOI] [PubMed] [Google Scholar]
- 6.Rechardt M, Shiri R, Karppinen J, Jula A, Heliövaara M, Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC Musculoskelet Disord. 2010;11:165. doi: 10.1186/1471-2474-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin TTL, Lin CH, Chang CL, Chi CH, Chang ST, Sheu WHH. The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med. 2015;43(9):2126–2132. doi: 10.1177/0363546515588173. [DOI] [PubMed] [Google Scholar]
- 8.Ranger TA, Wong AMY, Cook JL, Gaida JE. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sports Med. 2016;50(16):982–989. doi: 10.1136/bjsports-2015-094735. [DOI] [PubMed] [Google Scholar]
- 9.Longo UG, Franceschi F, Spiezia F, Forriol F, Maffulli N, Denaro V. Triglycerides and total serum cholesterol in rotator cuff tears: do they matter? Br J Sports Med. 2010;44(13):948–951. doi: 10.1136/bjsm.2008.056440. [DOI] [PubMed] [Google Scholar]
- 10.Akturk M, Karaahmetoglu S, Kacar M, Muftuoglu O. Thickness of the supraspinatus and biceps tendons in diabetic patients. Diabetes Care. 2002;25(2):408. doi: 10.2337/diacare.25.2.408. [DOI] [PubMed] [Google Scholar]
- 11.Yue DK, Swanson B, McLennan S, et al. Abnormalities of granulation tissue and collagen formation in experimental diabetes, uraemia and malnutrition. Diabet Med. 1986;3(3):221–225. doi: 10.1111/j.1464-5491.1986.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 12.Spanheimer RG. Correlation between decreased collagen production in diabetic animals and in cells exposed to diabetic serum: response to insulin. Matrix. 1992;12(2):101–107. doi: 10.1016/s0934-8832(11)80051-x. [DOI] [PubMed] [Google Scholar]
- 13.Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp Diabesity Res. 2004;5(2):143–153. doi: 10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi M, Tanigawa K, Tanaka O, Kato Y. Embryonic growth impaired by maternal hypoglycemia during early organogenesis in normal and diabetic rats. Acta Diabetol. 1994;31(3):141–146. doi: 10.1007/BF00570368. [DOI] [PubMed] [Google Scholar]
- 15.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103(6):2068–2076. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 16.Gooch HL, Hale JE, Fujioka H, Balian G, Hurwitz SR. Alterations of cartilage and collagen expression during fracture healing in experimental diabetes. Connect Tissue Res. 2000;41(2):81–91. doi: 10.3109/03008200009067660. [DOI] [PubMed] [Google Scholar]
- 17.Arkkila PE, Kantola IM, Viikari JS, Rönnemaa T. Shoulder capsulitis in type I and II diabetic patients: association with diabetic complications and related diseases. Ann Rheum Dis. 1996;55(12):907–914. doi: 10.1136/ard.55.12.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arkkila PE, Kantola IM, Viikari JS, Rönnemaa T, Vähätalo MA. Limited joint mobility is associated with the presence but does not predict the development of microvascular complications in type 1 diabetes. Diabet Med. 1996;13(9):828–833. doi: 10.1002/(SICI)1096-9136(199609)13:9<828∷AID-DIA182>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Balci N, Balci MK, Tüzüner S. Shoulder adhesive capsulitis and shoulder range of motion in type II diabetes mellitus: association with diabetic complications. J Diabetes Complicat. 1999;13(3):135–140. doi: 10.1016/s1056-8727(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 20.Cagliero E, Apruzzese W, Perlmutter GS, Nathan DM. Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am J Med. 2002;112(6):487–490. doi: 10.1016/s0002-9343(02)01045-8. [DOI] [PubMed] [Google Scholar]
- 21.Mavrikakis ME, Drimis S, Kontoyannis DA, Rasidakis A, Moulopoulou ES, Kontoyannis S. Calcific shoulder periarthritis (tendinitis) in adult onset diabetes mellitus: a controlled study. Ann Rheum Dis. 1989;48(3):211–214. doi: 10.1136/ard.48.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho CH, Kim DH, Lee YK. Serial Comparison of Clinical Outcomes After Arthroscopic Capsular Release for Refractory Frozen Shoulder With and Without Diabetes. Arthroscopy. 2016;32(8):1515–1520. doi: 10.1016/j.arthro.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Chen AL, Shapiro JA, Ahn AK, Zuckerman JD, Cuomo F. Rotator cuff repair in patients with type I diabetes mellitus. J Shoulder Elbow Surg. 2003;12(5):416–421. doi: 10.1016/S1058274603001721. [DOI] [PubMed] [Google Scholar]
- 24.Clement ND, Hallett A, MacDonald D, Howie C, McBirnie J. Does diabetes affect outcome after arthroscopic repair of the rotator cuff? J Bone Joint Surg Br. 2010;92(8):1112–1117. doi: 10.1302/0301-620X.92B8.23571. [DOI] [PubMed] [Google Scholar]
- 25.Dhar Y, Anakwenze OA, Steele B, Lozano S, Abboud JA. Arthroscopic rotator cuff repair: impact of diabetes mellitus on patient outcomes. Phys Sportsmed. 2013;41(1):22–29. doi: 10.3810/psm.2013.02.1995. [DOI] [PubMed] [Google Scholar]
- 26.Dutta U, Cohenford MA, Guha M, Dain JA. Non-enzymatic interactions of glyoxylate with lysine, arginine, and glucosamine: A study of advanced non-enzymatic glycation like compounds. Bioorganic Chemistry. 2007;35(1):11–24. doi: 10.1016/j.bioorg.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Gumucio JP, Korn MA, Saripalli AL, et al. Aging-associated exacerbation in fatty degeneration and infiltration following rotator cuff tear. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons. 2014;23(1) doi: 10.1016/j.jse.2013.04.011. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snedeker JG, Gautieri A. The role of collagen crosslinks in ageing and diabetes - the good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014;4(3):303–308. [PMC free article] [PubMed] [Google Scholar]
- 29.Fox AJS, Bedi A, Deng XH, et al. Diabetes mellitus alters the mechanical properties of the native tendon in an experimental rat model. J Orthop Res. 2011;29(6):880–885. doi: 10.1002/jor.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32(3-4):169–177. doi: 10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Valencia JV, Weldon SC, Quinn D, et al. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Analytical Biochemistry. 2004;324(1):68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Franke S, Sommer M, Rüster C, et al. Advanced glycation end products induce cell cycle arrest and proinflammatory changes in osteoarthritic fibroblast-like synovial cells. Arthritis Res Ther. 2009;11(5):R136. doi: 10.1186/ar2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handa A, Gotoh M, Hamada K, et al. Vascular endothelial growth factor 121 and 165 in the subacromial bursa are involved in shoulder joint contracture in type II diabetics with rotator cuff disease. J Orthop Res. 2003;21(6):1138–1144. doi: 10.1016/S0736-0266(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 34.Boivin GP, Elenes EY, Schultze AK, Chodavarapu H, Hunter SA, Elased KM. Biomechanical properties and histology of db/db diabetic mouse Achilles tendon. Muscles Ligaments Tendons J. 2014;4(3):280–284. [PMC free article] [PubMed] [Google Scholar]
- 35.Grant WP, Sullivan R, Sonenshine DE, et al. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg. 1997;36(4):272–278. doi: 10.1016/s1067-2516(97)80072-5. discussion 330. [DOI] [PubMed] [Google Scholar]
- 36.Chbinou N, Frenette J. Insulin-dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2004;286(5):R952–R957. doi: 10.1152/ajpregu.00536.2003. [DOI] [PubMed] [Google Scholar]
- 37.Bedi A, Fox AJS, Harris PE, et al. Diabetes mellitus impairs tendon-bone healing after rotator cuff repair. Journal of Shoulder and Elbow Surgery. 2010;19(7):978–988. doi: 10.1016/j.jse.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zakaria MHB, Davis WA, Davis TME. Incidence and predictors of hospitalization for tendon rupture in Type 2 diabetes: the Fremantle Diabetes Study. Diabet Med. 2014;31(4):425–430. doi: 10.1111/dme.12344. [DOI] [PubMed] [Google Scholar]
- 39.Cho NS, Moon SC, Jeon JW, Rhee YG. The Influence of Diabetes Mellitus on Clinical and Structural Outcomes After Arthroscopic Rotator Cuff Repair. Am J Sports Med. 2015;43(4):991–997. doi: 10.1177/0363546514565097. [DOI] [PubMed] [Google Scholar]
- 40.Blonna D, Fissore F, Bellato E, et al. Subclinical hypothyroidism and diabetes as risk factors for postoperative stiff shoulder. Knee Surg Sports Traumatol Arthrosc. 2015 Dec;:1–9. doi: 10.1007/s00167-015-3906-z. [DOI] [PubMed] [Google Scholar]
- 41.Chen AL, Shapiro JA, Ahn AK, Zuckerman JD, Cuomo F. Rotator cuff repair in patients with type I diabetes mellitus. Journal of Shoulder and Elbow Surgery. 2003;12(5):416–421. doi: 10.1016/S1058-2746(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 42.Herrera MF, Bauer G, Reynolds F, Wilk RM, Bigliani LU, Levine WN. Infection after mini-open rotator cuff repair. Journal of Shoulder and Elbow Surgery. 2002;11(6):605–608. doi: 10.1067/mse.2002.127302. [DOI] [PubMed] [Google Scholar]
- 43.Hsu SL, Ko JY, Chen SH, Wu RW, Chou WY, Wang CJ. Surgical Results in Rotator Cuff Tears with Shoulder Stiffness. Journal of the Formosan Medical Association. 2007;106(6):452–461. doi: 10.1016/S0929-6646(09)60294-1. [DOI] [PubMed] [Google Scholar]
- 44.Namdari S, Green A. Range of motion limitation after rotator cuff repair. Journal of Shoulder and Elbow Surgery. 2010;19(2):290–296. doi: 10.1016/j.jse.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Vasan S, Zhang X, Zhang X, et al. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996;382(6588):275–278. doi: 10.1038/382275a0. [DOI] [PubMed] [Google Scholar]
- 46.Rizvi AA, Chillag SA, Chillag KJ. Perioperative management of diabetes and hyperglycemia in patients undergoing orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(7):426–435. doi: 10.5435/00124635-201007000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty Infiltration and Atrophy of the Rotator Cuff Do Not Improve After Rotator Cuff Repair and Correlate With Poor Functional Outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 48.Beeharry D, Coupe B, Benbow EW, et al. Familial hypercholesterolaemia commonly presents with Achilles tenosynovitis. Ann Rheum Dis. 2006;65(3):312–315. doi: 10.1136/ard.2005.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von S Bahr, Movin T, Papadogiannakis N, et al. Mechanism of Accumulation of Cholesterol and Cholestanol in Tendons and the Role of Sterol 27-hydroxylase (CYP27A1) Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;;22(7):1129–1135. doi: 10.1161/01.ATV.0000022600.61391.A5. [DOI] [PubMed] [Google Scholar]
- 50.Ozgurtas T, Yildiz C, Serdar M, Atesalp S, Kutluay T. Is high concentration of serum lipids a risk factor for Achilles tendon rupture? Clin Chim Acta. 2003;331(1-2):25–28. doi: 10.1016/s0009-8981(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 51.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 1984;4(4):323–340. doi: 10.1161/01.ATV.4.4.323. [DOI] [PubMed] [Google Scholar]
- 52.Mendias CL, Roche SM, Harning JA, et al. Reduced muscle fiber force production and disrupted myofibril architecture in patients with chronic rotator cuff tears. J Shoulder Elbow Surg. 2015;24(1):111–119. doi: 10.1016/j.jse.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 53.Davis ME, Korn MA, Gumucio JP, et al. Simvastatin reduces fibrosis and protects against muscle weakness after massive rotator cuff tear. J Shoulder Elbow Surg. 2015;24(2):280–287. doi: 10.1016/j.jse.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010;468(6):1493–1497. doi: 10.1007/s11999-009-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 56.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(6):550–554. doi: 10.1016/S1058274603002118. [DOI] [PubMed] [Google Scholar]
- 57.Beason DP, Tucker JJ, Lee CS, Edelstein L, Abboud JA, Soslowsky LJ. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. Journal of Shoulder and Elbow Surgery. 2014;23(6):867–872. doi: 10.1016/j.jse.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaida JE, Cook JL, Bass SL. Adiposity and tendinopathy. Disability and Rehabilitation. 2008;30(20-22):1555–1562. doi: 10.1080/09638280701786864. [DOI] [PubMed] [Google Scholar]
- 59.Soslowsky LJ, Fryhofer GW. Tendon Homeostasis in Hypercholesterolemia. Adv Exp Med Biol. 2016;920:151–165. doi: 10.1007/978-3-319-33943-6_14. [DOI] [PubMed] [Google Scholar]
- 60.Józsa L, Réffy A, Bálint JB. The pathogenesis of tendolipomatosis; an electron microscopical study. Int Orthop. 1984;7(4):251–255. doi: 10.1007/BF00266836. [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto F, Uhthoff HK, Trudel G, Loehr JF. Delayed tendon reattachment does not reverse atrophy and fat accumulation of the supraspinatus--an experimental study in rabbits. J Orthop Res. 2002;20(2):357–363. doi: 10.1016/S0736-0266(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 62.Killian Ml, Lim Ct, Thomopoulos S, Charlton N, Kim Hm, Galatz Lm. The effect of unloading on gene expression of healthy and injured rotator cuffs. J Orthop Res. 2013;31(8):1240–1248. doi: 10.1002/jor.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung SW, Park H, Kwon J, Choe GY, Kim SH, Oh JH. Effect of Hypercholesterolemia on Fatty Infiltration and Quality of Tendon-to-Bone Healing in a Rabbit Model of a Chronic Rotator Cuff Tear Electrophysiological, Biomechanical, and Histological Analyses. Am J Sports Med. 2016;44(5):1153–1164. doi: 10.1177/0363546515627816. [DOI] [PubMed] [Google Scholar]
- 64.Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014;23(4):445–455. doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 65.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A(9):1973–1982. doi: 10.2106/00004623-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Beason DP, Hsu JE, Marshall SM, et al. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg. 2013;22(5):681–686. doi: 10.1016/j.jse.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JM, Kim MW, Do HJ. Influence of Hyperlipidemia on the Treatment of Supraspinatus Tendinopathy With or Without Tear. Annals of Rehabilitation Medicine. 2016;40(3):463. doi: 10.5535/arm.2016.40.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 69.de Oliveira LP, Vieira CP, Guerra FD, Almeida MS, Pimentel ER. Structural and biomechanical changes in the Achilles tendon after chronic treatment with statins. Food Chem Toxicol. 2015;77:50–57. doi: 10.1016/j.fct.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Tucker JJ, Soslowsky LJ. Effect of simvastatinon rat supraspinatus tendon mechanical and histological properties in a diet-induced hypercholesterolemia model. J Orthop Res. 2016 Mar; doi: 10.1002/jor.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dolkart O, Liron T, Chechik O, et al. Statins Enhance Rotator Cuff Healing by Stimulating the COX2/PGE2/EP4 Pathway An In Vivo and In Vitro Study. Am J Sports Med. 2014;42(12):2869–2876. doi: 10.1177/0363546514545856. [DOI] [PubMed] [Google Scholar]
- 72.Oliva F, Via AG, Maffulli N. Calcific tendinopathy of the rotator cuff tendons. Sports Med Arthrosc. 2011;19(3):237–243. doi: 10.1097/JSA.0b013e318225bc5f. [DOI] [PubMed] [Google Scholar]
- 73.Milgrom C, Novack V, Weil Y, Jaber S, Radeva-Petrova DR, Finestone A. Risk factors for idiopathic frozen shoulder. Isr Med Assoc J. 2008;10(5):361–364. [PubMed] [Google Scholar]
- 74.Bassett JHD, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213(1):1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 75.Knopp WD, Bohm ME, McCoy JC. Hypothyroidism Presenting as Tendinitis. The Physician and Sportsmedicine. 1997;25(1):47–55. doi: 10.3810/psm.1997.01.1094. [DOI] [PubMed] [Google Scholar]
- 76.Harvie P, Pollard TCB, Carr AJ. Calcific tendinitis: Natural history and association with endocrine disorders. Journal of Shoulder and Elbow Surgery. 2007;16(2):169–173. doi: 10.1016/j.jse.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Pantazis K, Roupas ND, Panagopoulos A, Theodoraki S, Tsintoni A, Kyriazopoulou V. Spontaneous rupture of the long head of the biceps tendon in a woman with hypothyroidism: a case report. J Med Case Rep. 2016;10:2. doi: 10.1186/s13256-015-0794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flamant F, Samarut J. Thyroid hormone receptors: lessons from knockout and knock-in mutant mice. Trends Endocrinol Metab. 2003;14(2):85–90. doi: 10.1016/s1043-2760(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 79.Anwar S, Gibofsky A. Musculoskeletal manifestations of thyroid disease. Rheum Dis Clin North Am. 2010;36(4):637–646. doi: 10.1016/j.rdc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Oliva F, Berardi AC, Misiti S, Falzacappa CV, Iacone A, Maffulli N. Thyroid hormones enhance growth and counteract apoptosis in human tenocytes isolated from rotator cuff tendons. Cell Death and Disease. 2013;4(7):e705. doi: 10.1038/cddis.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 82.Oliva F, Berardi AC, Misiti S, Maffulli N. Thyroid hormones and tendon: current views and future perspectives. Concise review. Muscles Ligaments Tendons J. 2013;3(3):201–203. [PMC free article] [PubMed] [Google Scholar]
- 83.Purnell DC, Daly DD, Lipscomb PR. Carpal-tunnel syndrome associated with myxedema. Arch Intern Med. 1961;108:751–756. doi: 10.1001/archinte.1961.03620110091012. [DOI] [PubMed] [Google Scholar]
- 84.Berardi AC, Oliva F, Berardocco M, La Rovere M, Accorsi P, Maffulli N. Thyroid hormones increase collagen I and cartilage oligomeric matrix protein (COMP) expression in vitro human tenocytes. Muscles Ligaments Tendons J. 2014;4(3):285–291. [PMC free article] [PubMed] [Google Scholar]
- 85.Oliva F, Osti L, Padulo J, Maffulli N. Epidemiology of the rotator cuff tears: a new incidence related to thyroid disease. Muscles, Ligaments and Tendons Journal. 2014;4(3):309. [PMC free article] [PubMed] [Google Scholar]
- 86.Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123(2):183.e1–9. doi: 10.1016/j.amjmed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 87.Oliva F, Zocchi L, Codispoti A, et al. Transglutaminases expression in human supraspinatus tendon ruptures and in mouse tendons. Biochem Biophys Res Commun. 2009;379(4):887–891. doi: 10.1016/j.bbrc.2008.12.188. [DOI] [PubMed] [Google Scholar]
- 88.Lee JJ, Patel R, Biermann JS, Dougherty PJ. The Musculoskeletal Effects of Cigarette Smoking. J Bone Joint Surg Am. 2013;95(9):850–859. doi: 10.2106/JBJS.L.00375. [DOI] [PubMed] [Google Scholar]
- 89.Kanneganti P, Harris JD, Brophy RH, Carey JL, Lattermann C, Flanigan DC. The Effect of Smoking on Ligament and Cartilage Surgery in the Knee A Systematic Review. Am J Sports Med. 2012;40(12):2872–2878. doi: 10.1177/0363546512458223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baumgarten KM, Gerlach D, Galatz LM, et al. Cigarette smoking increases the risk for rotator cuff tears. Clin Orthop Relat Res. 2010;468(6):1534–1541. doi: 10.1007/s11999-009-0781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. 2012;255(6):1069–1079. doi: 10.1097/SLA.0b013e31824f632d. [DOI] [PubMed] [Google Scholar]
- 92.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28(1):1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 93.Katzer A, Wening JV, Becker-Männich HU, Lorke DE, Jungbluth KH. Rotator cuff rupture. Vascular supply and collagen fiber processes as pathogenetic factors. Unfallchirurgie. 1997;23(2):52–59. doi: 10.1007/BF02628150. [DOI] [PubMed] [Google Scholar]
- 94.Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med. 2012;31(4):589–604. doi: 10.1016/j.csm.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N, Thomopoulos S. Nicotine Delays Tendon-to-Bone Healing in a Rat Shoulder Model. J Bone Joint Surg Am. 2006;88(9):2027–2034. doi: 10.2106/JBJS.E.00899. [DOI] [PubMed] [Google Scholar]
- 96.Jorgensen LN, Kallehave F, Christensen E, Siana JE, Gottrup F. Less collagen production in smokers. Surgery. 1998;123(4):450–455. doi: 10.1016/S0039-6060(98)70167-9. [DOI] [PubMed] [Google Scholar]
- 97.Ichinose R, Sano H, Kishimoto KN, Sakamoto N, Sato M, Itoi E. Alteration of the material properties of the normal supraspinatus tendon by nicotine treatment in a rat model. Acta Orthopaedica. 2010;81(5):634–638. doi: 10.3109/17453674.2010.524595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ling SC, Chen CF, Wan RX. A study on the vascular supply of the supraspinatus tendon. Surg Radiol Anat. 1990;12(3):161–165. doi: 10.1007/BF01624517. [DOI] [PubMed] [Google Scholar]
- 99.Determe D, Rongières M, Kany J, et al. Anatomic study of the tendinous rotator cuff of the shoulder. Surg Radiol Anat. 18(3):195–200. doi: 10.1007/BF02346127. [DOI] [PubMed] [Google Scholar]
- 100.Daftari TK, Whitesides TE, Heller JG, Goodrich AC, McCarey BE, Hutton WC. Nicotine on the revascularization of bone graft. An experimental study in rabbits. Spine. 1994;19(8):904–911. doi: 10.1097/00007632-199404150-00007. [DOI] [PubMed] [Google Scholar]
- 101.Lundgreen K, Lian ØB, Scott A, Nassab P, Fearon A, Engebretsen L. Rotator Cuff Tear Degeneration and Cell Apoptosis in Smokers Versus Nonsmokers. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2014;30(8):936–941. doi: 10.1016/j.arthro.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neyton L, Godenèche A, Nové-Josserand L, Carrillon Y, Cléchet J, Hardy MB. Arthroscopic suture-bridge repair for small to medium size supraspinatus tear: healing rate and retear pattern. Arthroscopy. 2013;29(1):10–17. doi: 10.1016/j.arthro.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 103.Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13–20. doi: 10.1016/j.jse.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 104.Tashjian RZ, Hollins AM, Kim HM, et al. Factors Affecting Healing Rates After Arthroscopic Double-Row Rotator Cuff Repair. Am J Sports Med. 2010;38(12):2435–2442. doi: 10.1177/0363546510382835. [DOI] [PubMed] [Google Scholar]
- 105.Carbone S, Gumina S, Arceri V, Campagna V, Fagnani C, Postacchini F. The impact of preoperative smoking habit on rotator cuff tear: cigarette smoking influences rotator cuff tear sizes. Journal of Shoulder and Elbow Surgery. 2012;21(1):56–60. doi: 10.1016/j.jse.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 106.Balyk R, Luciak-Corea C, Otto D, Baysal D, Beaupre L. Do outcomes differ after rotator cuff repair for patients receiving workers' compensation? Clin Orthop Relat Res. 2008;466(12):3025–3033. doi: 10.1007/s11999-008-0475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Almeida A, Valin MR, Zampieri R, de Almeida NC, Roveda G, Agostini AP. Comparative Analysis On The Result For Arthroscopic Rotator Cuff Suture Between Smoking And Non-Smoking Patients. Rev Bras Ortop. 2011;46(2)(15):172–175. 30235–4. doi: 10.1016/S2255-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prasad N, Odumala A, Elias F, Jenkins T. Outcome of open rotator cuff repair. An analysis of risk factors. Acta Orthop Belg. 2005;71(6):662–666. [PubMed] [Google Scholar]


