Abstract
Context:
Biochemical changes occur in biological fluids and tissues of different types of malignancies. Tumor markers in serum, tissue, and other body fluids during neoplastic process are of clinical value in the management of patients with cancers. Serum alkaline phosphatase (ALP) activity is potentially a useful indicator for detection of malignancies, but its status in oral squamous cell carcinoma (OSCC) is less explored.
Aims:
The aim of this study is to evaluate the serum level of ALP in OSCC patients and assess its relation with the clinicopathological features.
Settings and Design:
A total of 175 participants (145 OSCC patients and 30 healthy controls) were included in the study. One hundred and forty-five patients with OSCC who underwent treatment at our institution were included to obtain the clinicopathological data.
Materials and Methods:
Fasting blood ALP activity was evaluated using ALP assessment kit and biochemistry analyzer.
Statistical Analysis Used:
The data were analyzed by SPSS-21 software (SPSS Statistics for Windows, Version 21.0, Armonk, NY, USA), using t-test, Mann–Whitney U, and Kruskal–Wallis tests.
Results:
Raised ALP was seen in 24% of OSCC patients. The mean ALP in OSCC was significantly higher than the control. ALP level in patients with advanced stage was significantly higher than with early stage. The serum ALP level in OSCC patients with bone involvement (BI) by local extension of tumor was significantly higher than without BI.
Conclusion:
ALP showed statistically significant differences in relation to tumor stages and BI. Hence, ALP could be useful in advanced stage disease for expressing the endurance of patient and tumor expansion. Elevated ALP in OSCC patients may indicate BI.
Keywords: Alkaline phosphatase, clinicopathological parameters, oral squamous cell carcinoma
Introduction
Alkaline phosphatase (ALP) comprises a group of enzymes that catalyze the hydrolysis of phosphate esters in an alkaline environment.[1] In healthy adults, ALP is mainly derived from the liver and bones and in lesser amounts from intestines, placenta, kidneys, and leukocytes. Although the exact metabolic function of enzyme is not yet understood, it appears that ALP is associated with lipid transport in the intestine and with the calcification process in the bone.[2] ALP is also responsible for cleaving phosphate groups from other molecules such as nucleotides which are building blocks for DNA and proteins.[3]
Serum ALP levels are elevated in patients with primary and metastatic tumors of the liver and bone, such as hepatic metastasis of colorectal cancer and bone and liver involvement in breast cancer.[1,4] In patients with malignancies, therefore, an elevated serum ALP may be an indicator of metastatic disease.[1] However, primary cancers in various organs can generate ALP elevations in the absence of metastasis.[5] Although the association between serum levels of ALP and systemic metastases in malignancies has been demonstrated, the correlation between elevation of ALP and lymph node metastasis (LNM) has not been studied extensively. Regional lymph nodes are generally the first sites of tumor cell involvement outside the primary malignant focus in most solid tumors. In addition, no serum marker predictive of LNM has been suggested yet.[1] Moreover, the role of ALP in oral squamous cell carcinoma (OSCC) is barely investigated. Hence, the objectives of the study were to evaluate the serum ALP activity in OSCC patients and their association with clinicopathological parameters. The study is unique as it intended to analyze if ALP vary with jaw bone involvement (BI) by the local extension of primary tumor.
Materials and Methods
A total of 175 patients ranging in age from 20 to 75 years were included in this case–control study after obtaining approval by the Ethical Committee of the institute (IRB No. 2015/S/OP/34). After obtaining written informed consent from the participants, thorough demographic and clinical details were recorded. The participants were divided into: Group I consisted of 145 untreated OSCC patients, reporting to the institution for treatment (excision of the lesion along with neck dissection). The patients were diagnosed as having OSCC based on clinical and histopathological examinations. Staging was done according to the Union for International Cancer Control classification. Complete clinical data including age, gender, habits, tumor location and extension, growth pattern, size, and clinical nodal status were recorded along with findings of imaging investigations done to make out the extension of the lesion. Preoperative fasting blood ALP activity was evaluated along with the complete blood investigation which was routinely done before surgery. As a part of patients’ workup before treatment, followings are the investigations routinely done: complete hemogram, bleeding time, clotting time, total protein, albumin, globulin, blood glucose, urea, creatinine, bilirubin, SGOT, SGPT, lipid profile, and electrolyte (Na, K, and Cl). Postoperatively, complete clinicopathological data, including type of lesion, tumor size, staging, grade, and lymph nodal status, were obtained from patients’ surgically excised specimen.
Group II included thirty age- and sex-matched healthy individuals who visited the institution for routine dental checkup and/treatment, without any history of tobacco use and oral lesions. These participants were selected randomly among the people who visited the same hospital during the same period.
Patients with history of diabetes, hypertension, cardiac, liver, renal, and bone diseases were excluded from the study. None of the participants included in the study were on statins or glitazones.
Estimation of serum alkaline phosphatase activity
ALP assessment kit was used with a technique in accordance with the recommendations of the International Federation of Clinical Chemistry with the help of biochemistry analyzer (Erba Chem 5 Plus v2; ERBA Diagnostics Mannheim GmbH, Mannheim, Germany). Under all aseptic precautions, 5 mL fasting venous blood was collected from antecubital vein of study and control group into plain sterile bulb. The sample was then allowed to clot at room temperature and then centrifuged at 3000 rpm for 10 min to separate the serum. ALP levels were measured using spectrophotometer with cell holder thermostable at 37°C, along with a commercially available ALP assessment kit. ALP values were determined by the end-point spectrophotometric method. Hydrolysis of 4-nitrophenyl phosphate in the alkaline substrate produces 4-nitrophenyl of yellow color whose intensity is determined on spectrophotometer at a wavelength of 405 nm. The activity of ALP was calculated with molar extinction coefficient, and results of the enzyme activity were given in the international units (IUs).[6] The normal range is 20–140 IU/L.[7]
Statistical analysis
The level of ALP in IU/L was presented as the mean and standard deviation. Evaluation of results and statistical analysis was carried out using t-test, Mann–Whitney U, and Kruskal–Wallis tests. A value of P < 0.05 was considered significant. All statistical analyses were conducted IBM Corp., SPSS Statistics for Windows, Version 21.0, Armonk, NY, USA.
Results
Alkaline phosphatase activity in serum of healthy controls
Total ALP activity in sera of healthy controls was in the range of 58–123 IU/L (median 81 IU/L).
Alkaline phosphatase activity in serum of oral squamous cell carcinoma patients
The total ALP activity in all patients with malignant disease was in the range of 54–271 IU/L (median 110 IU/L). Raised ALP was seen in 24% of OSCC patients. Table 1 shows the mean value of ALP in OSCC to be higher than the control and was significant (P = 0.000).
Table 1.
Distribution of mean serum alkaline phosphatase level in oral squamous cell carcinoma and control group

Clinicopathological characteristics of oral squamous cell carcinoma patients
The mean age of 145 patients with OSCC was 48.11 years. Sixty-one percent of patients were ≥45 years, and 83% of patients were males. Primarily buccal mucosa was involved in 72% cases followed by tongue (16%) and gingiva (12%). Most of the patients treated were from low socioeconomic background. Sixty-two percent (90) of patients were chronic pan-tobacco chewers consequently presenting with tumor involving gingivobuccal complex. Combination of habits was reported in 38% (55) OSCC patients. Of which, nearly thirty patients gave a history of alcohol consumption thrice a week to every day for 8–12 years, resulting in tumors involving tongue and floor of mouth. Forty percent of tumors were >4 cm, and 61% of lesions were in advanced stage. Seventy-nine percent of tumors exhibited an exophytic growth. Forty-six percent had LNM. BI due to local extension of the primary tumor was noted in 25% cases. BI was confirmed by imaging modalities and grossing of the excised tumor specimen and histopathologic evaluation.
The mean ALP values increased from early to advanced stage, and this was found to be statistically significant. The mean ALP in OSCC patients with considerable BI by local extension of tumor was significantly higher than without BI. There was no statistically significant relation between the ALP level and various clinicopathological parameters [Table 2]. However, the observations reveal that mean value of ALP was higher in tumors involving multiple sites than a single site in tumor size >4 cm and <4 cm and in tumors with LNM and without LNM.
Table 2.
Alkaline phosphatase level and clinicopathological parameters in patients with oral squamous cell carcinoma (n=145)
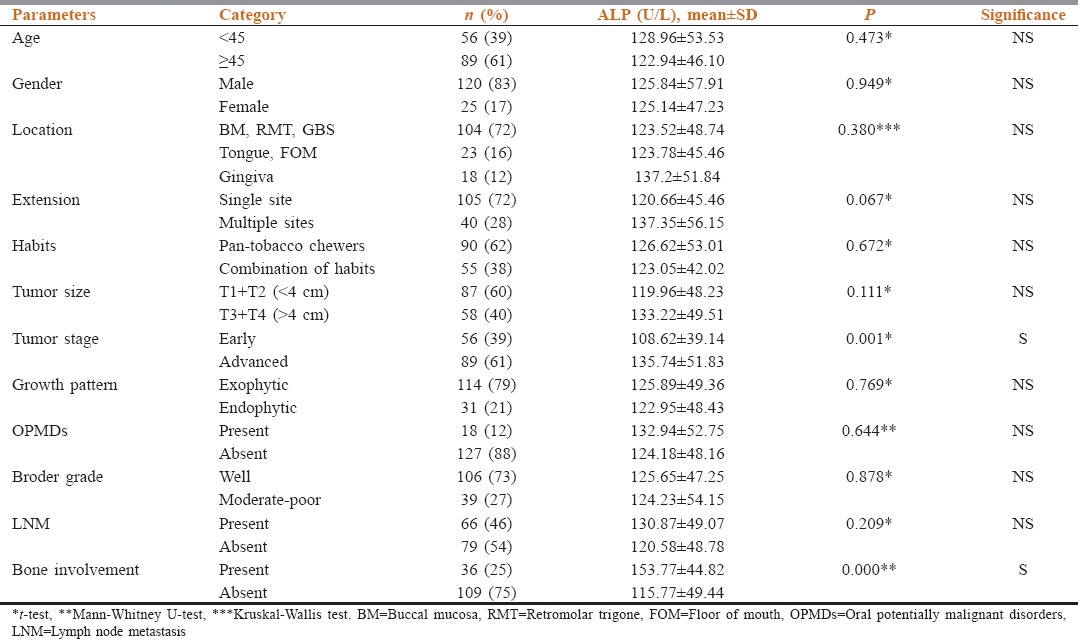
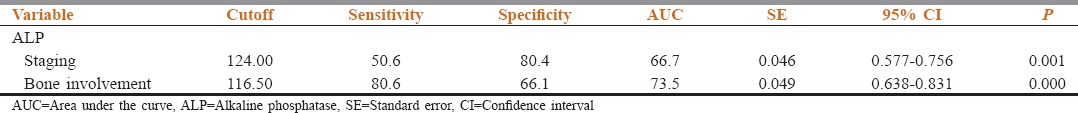
Analyses by receiver-operating characteristic (ROC) curves using ALP to make (a) the clinical assessment regarding staging and (b) the clinical decision regarding BI were done. The areas under the ROC curve were 0.667 and 0.735, respectively [Figure 1a and b]. The cutoff point for maximum specificity and sensitivity was established as 124 IU/L using ROC analysis. Patients with ALP >124 IU/L were more likely to have advanced stage disease than patients with <124 IU/L. The cutoff point for maximum specificity and sensitivity was established as 116.5 IU/L using ROC analysis. Patients with an ALP >116.5 IU/L were more likely to have substantial BI by the local extension of tumor than patients with <116.5 IU/L [Table 3].
Figure 1.
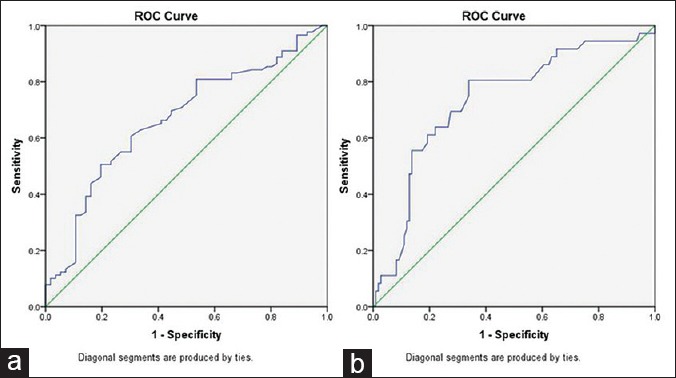
Receiver-operating characteristic curves for (a) alkaline phosphatase used to make the clinical decision regarding tumor staging; (b) alkaline phosphatase used to make the clinical decision regarding bone involvement. The areas under the receiver-operating characteristic curve were 0.667 and 0.735, respectively
Table 3.
Area under the curve and cutoff values obtained in receiver operating characteristic curve analysis
Discussion
OSCC encompasses at least 90% of all oral malignancies. OSCC grows locally and then it spreads to the lymph nodes of the neck. The therapeutic modality currently offered to OSCC is based on traditional stage-predicting indices, TNM criteria, and on histological grading. Pretreatment staging of patients with cancer has a major role in decision-making about care. This is generally achieved using imaging techniques. The results of current imaging techniques have enhanced the sensitivity and accuracy of node detection, but they are not perfect.[8] Thus, there has been an ever-growing effort dedicated to the basic research of oral cancer, focusing on the identification of biological indicators for the diagnosis of its biological nature and aggressiveness.[9]
Carcinogenesis leads to various biochemical changes in the body.[5] Biochemical parameter such as ALP evaluation is an inexpensive and potential marker for early detection of cancer that helps diagnosis.[4] Most data indicate that the elevation of serum ALP occurs because of the accelerated de novo synthesis of the enzyme and subsequent regurgitation into the serum.[10] Elevated levels of ALP are frequently observed in advanced cancers.[5] Ehrmeyer et al.,[11] Van Hoof VO et al.,[12] and Banseria et al. found raised serum ALP (<0.05) in head and neck cancers. The mean value of ALP in OSCC was higher than the control and was statistically significant. In the present study, raised ALP was seen in 24% of OSCC patients. The higher incidence of raised ALP found in our series could be due to the fact that majority of patients 61% included in the study have advanced stage lesion and with substantial BI in 25% cases.
There is a distinct difference in the site predilection of OSCC in the Indian population as compared to the Western population. The tobacco/quid is kept in gingivobuccal vestibule and thus carcinoma develops at this site more often than the other parts of the oral cavity. The precipitation of OSCC in the gingivobuccal vestibule or buccal mucosa tends to invade the underlying bone earlier than other sites.[13] The incidence of mandibular BI ranges from 12% to 56%.[14] In the present study, BI due to local extension of the primary tumor was noted in 25% cases. BI was confirmed by imaging modalities, grossing, and histologic examination of the excised tumor specimen. The mean ALP in OSCC patients with BI by local extension of tumor was significantly higher than without BI thus supporting the opinion proposed by Merza et al. that elevated ALP in OSCC may be due to bone destruction.[15] Hence, elevated ALP in patients with OSCC may be indicative of substantial BI by the tumor.
Banseria et al., in a study, to find the relation between ALP levels with grading and staging OSCC observed raised ALP in 8% cancer patients. The mean serum ALP value raised from Stage I-IV cancer and this was found to be statistically significant. In the present investigation, the mean ALP level in patients with advanced stage was significantly higher than with early stage. Similar results were obtained by Stolbach and Bassalyk, explaining the ectopic production of ALP at tumor site. Authors conclude that ALP is valuable in advanced disease for indicating survival and progress. Further studies are needed to know the ALP isoenzyme patterns in malignant diseases.[5] Application of techniques of isoenzyme analysis to the characterization of ALP in serum led to the discovery that forms of the enzymes essentially identical to normal placental isoenzyme appear in the sera of some patients with malignant disease.[2]
The presence of neoplastic tissue occasionally results in the release of tumor-associated proteins into the blood, such as carcinoembryonic antigen, alpha-fetoprotein, placental lactogen, and placental alkaline phosphatase isoenzymes. Electrophoretic form of ALP (FHAP) is associated with neoplasms of a variety of organ sites such as colon, breast as well as cell types such as adenocarcinoma, squamous cell carcinoma, and leukemia.[11] Prospective studies to evaluate FHAP need to be undertaken to recognize its importance in OSCC.
Cancer patients with bone metastasis mainly had elevated values of bone isoform ALP. Elevated ALP was due to bone destruction and compensation of osteoblastic activity mediated by an increased production of inflammatory mediators such as TNF-alpha.[6] In the present study, BI due to local extension of the primary tumor was noted in 25% cases. The mean ALP in OSCC patients with BI by local extension of tumor was significantly higher than without BI. Future studies of bone isoform ALP would give clarity with this regard.
Aminian et al. retrospectively examined serum ALP in esophageal carcinoma and found that mean ALP was higher in patients with LNM (141 U/L) than with negative node involvement (116 U/L), with a mean difference of (25 U/L). Thus, elevated ALP levels in patients with esophageal cancer may predict the LNM. In the present investigation, the mean ALP values in cases with LNM were higher than without LNM, but this was statistically insignificant.[1]
Conclusion
To the best of our knowledge, this is the first of its kind study to assess the relationship between ALP with various clinicopathological features. The present study and previous reports showed that in cancer patients the total ALP enzyme activity was higher than the activity in healthy people of the same age. The result suggests that ALP exhibits variation with respect to parameters such as tumor extension, size, stage, LNM, and BI status, thus confirming its role as tumor marker. Further studies with larger sample size should be undertaken to validate the present findings and to analyze the isoenzyme activity in OSCC. Prospective studies need to focus on the usefulness of tumor ALP in OSCC. Identification of tumor-related variants early in the course of disease and their significance needs to be elucidated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Aminian A, Karimian F, Mirsharifi R, Alibakhshi A, Hasani SM, Dashti H, et al. Correlation of serum alkaline phosphatase with clinicopathological characteristics of patients with oesophageal cancer. East Mediterr Health J. 2011;17:862–6. doi: 10.26719/2011.17.11.862. [DOI] [PubMed] [Google Scholar]
- 2.Panteghini M, Bais R. Serum enzymes. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. Philadelphia: Elsevier Saunders; 2012. pp. 577–9. [Google Scholar]
- 3.Friedman LS, Martin P, Munoz SJ. Liver function tests and the objective evaluation of the patient with liver disease. In: Zakim D, Boyer TD, editors. Hepatology: A Textbook of Liver Disease. Philadelphia: WB Saunders; 1996. pp. 791–833. [Google Scholar]
- 4.Saif MW, Alexander D, Wicox CM. serum alkaline phosphatase level as a prognostic tool in colorectal cancer: A study of 105 patients. J Appl Res. 2005;5:88–95. [PMC free article] [PubMed] [Google Scholar]
- 5.Banseria N, Malik R, Nigam RK, Trichal VK, Shrivastava A, Jain R, et al. Correlation of serum lipid profile, serum calcium, alkaline phosphatase and serum protein with histopathological grading and staging in head and neck cancer. J Evol Med Dent Sci. 2014;3:1978–86. [Google Scholar]
- 6.Dokic-Lisanin M, Pantovic V, Jovanovic Z, Samardzic G, Jurisic V. Values of alkaline phosphatase and their isoenzyme profiles in patients with cancer in respect to bone and liver metastasis. Arch Oncol. 2013;21:14–6. [Google Scholar]
- 7.Abd El Maksoud N, Samy N, Ragab HM, Shaalan M. Clinical significance of antioxidants, lactate dehydrogenase and alkaline phosphates in breast cancer patients with and without lymph node metastases. J Genet Eng Biotechnol. 2009;7:59–64. [Google Scholar]
- 8.Martínez-Gimeno C, Rodríguez EM, Vila CN, Varela CL. Squamous cell carcinoma of the oral cavity: A clinicopathologic scoring system for evaluating risk of cervical lymph node metastasis. Laryngoscope. 1995;105(7 Pt 1):728–33. doi: 10.1288/00005537-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Nagler R, Bahar G, Shpitzer T, Feinmesser R. Concomitant analysis of salivary tumor markers – A new diagnostic tool for oral cancer. Clin Cancer Res. 2006;12:3979–84. doi: 10.1158/1078-0432.CCR-05-2412. [DOI] [PubMed] [Google Scholar]
- 10.Derlin TM. Text Book of Biochemistry with Clinical Correlations. 4th ed. New York: Wiley-Liss; 1997. pp. 343–6. [Google Scholar]
- 11.Ehrmeyer SL, Joiner BL, Kahan L, Larson FC, Metzenberg RL. A cancer-associated, fast, homoarginine-sensitive electrophoretic form of serum alkaline phosphatase. Cancer Res. 1978;38:599–601. [PubMed] [Google Scholar]
- 12.Van Hoof VO, Van Oosterom AT, Lepoutre LG, De Broe ME. Alkaline phosphatase isoenzyme patterns in malignant disease. Clin Chem. 1992;38:2546–51. [PubMed] [Google Scholar]
- 13.Nimonkar PV, Borle RM. Hypercalcemia in patients of oral squamous cell carcinoma. J Maxillofac Oral Surg. 2009;8:230–2. doi: 10.1007/s12663-009-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey M, Rao LP, Das SR, Mathews A, Chacko EM, Naik BR. Patterns of mandibular invasion in oral squamous cell carcinoma of the mandibular region. World J Surg Oncol. 2007;5:12. doi: 10.1186/1477-7819-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merza KS, Alaaraji SB, Abdullah BH. Comparative study on lactate dehydrogenase, alkaline phosphatase and immunoglobulins in serum and saliva of acute leukemia and oral squamous cell carcinoma patients. Iraqi J Sci. 2010;51:262–70. [Google Scholar]


