Fig. 5.
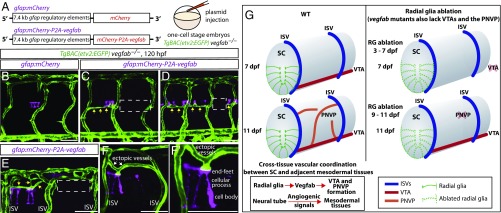
Mosaic overexpression of Vegfab in radial glia is sufficient to partially rescue the loss of VTAs in vegfab mutants and induce ectopic blood vessels directed toward their endfeet. (A) Schematic diagram of the gfap:mCherry and gfap:mCherry-P2A-vegfab plasmid injection experiments (B–F′). (B–F′) 120 hpf TgBAC(etv2:EGFP) trunks of vegfab−/− fish injected with the gfap:mCherry (B) or gfap:mCherry-P2A-vegfab (C–F′) plasmid. Partial recovery of VTAs was observed in the vegfab−/− fish injected with the gfap:mCherry-P2A-vegfab plasmid (yellow arrows, C and D), while VTAs were absent in the vegfab−/− fish injected with the gfap:mCherry plasmid (B). As observed in the regions inside the dashed lines (C and D), VTAs were absent in the somites where mCherry+ radial glia were not present in close proximity and/or sufficient numbers. Ectopic blood vessel sprouting toward mCherry+ radial glia overexpressing Vegfab (yellow arrows, E) was not observed in somites lacking mCherry+ radial glia (e.g., the region inside the dashed white line in E). Ectopic vessels (white arrows) were directed toward the endfeet of mCherry+ radial glia overexpressing Vegfab (F and F′). (Scale bar, 50 µm.) (G) Schematic diagrams showing radial glia regulation of angiogenesis around the developing spinal cord. During development, radial glia endfeet lie in close proximity to the forming VTAs. Radial glia ablation in early larval stages leads to the loss of the VTAs. Radial glia ablation at later stages impairs PNVP formation. vegfab mutants phenocopy the vascular defects observed in radial glia-ablated larvae. Thus, radial glia act as positive angiogenic regulators in mesodermal tissues around the spinal cord, which in turn allows spinal cord vascularization. Vessels are shown only on one side of the spinal cord. RG, radial glia; SC, spinal cord.