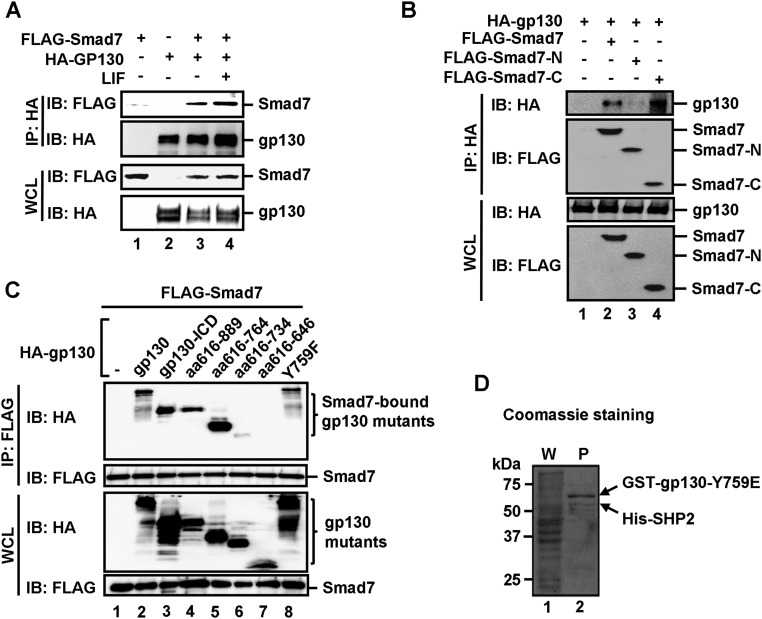
Fig. S5.
Smad7 directly binds to the cytoplasmic domain of gp130 and disrupts the binding of SHP2/SOCS3 to gp130. (A) Smad7 interacts with gp130 in vivo. HEK293T cells were cotransfected with FLAG–Smad7 and HA–gp130 and then treated with 1 ng/mL LIF for 2 h before harvest. The cell lysates were harvested and immunoprecipitated with anti-HA antibody. Smad7 and gp130 were detected from the immunoprecipitates by Western blot analysis. (B) The MH2 domain (or C domain) of Smad7 binds to gp130. HEK293T cells were transfected with indicated plasmids. Levels of these proteins in IP products and whole cell lysates were analyzed by Western blot analysis. (C) The 734–764 aa region in the cytoplasmic domain of gp130 is essential in Smad7 binding. HEK293T cells were cotransfected with FLAG–Smad7 and a HA-tagged gp130 wild-type or a deletion mutant, and then treated with 1 ng/mL LIF for 2 h before harvest. Cell lysates were harvested and immunoprecipitated with anti-FLAG antibody. Smad7 and gp130 variants were detected from the immunoprecipitates by Western blot analysis. (D) Formation of the Y759E–SHP2 complex in E. coli. Fusion proteins of GST–gp130–Y759E and His–SHP2 were coexpressed in E. coli. The Y759E–SHP2 complex was retrieved by glutathione Sepharose beads, as indicated by Coomassie staining. P, purified proteins on glutathione Sepharose beads; W, whole cellular protein lysates.