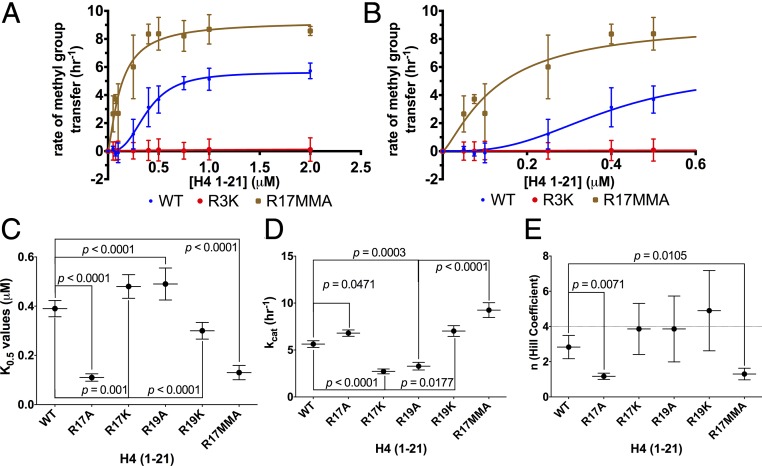
Fig. 2.
Monomethylation of H4 R17 affects the positive cooperativity exhibited by HsPRMT5/MEP50. Initial kinetic measurements were made, and the data were fit to the Hill equation (38). (A) Enzyme activity of HsPRMT5/MEP50 with H4 (1-21) WT (blue), H4 (1-21) R17MMA (brown), and H4 (1-21) R3K (red) is shown for triplicate assays (error bars represent SD). (B) An expanded view of A at the low substrate concentrations. Best fit curves are shown for K0.5, kcat, and Hill coefficient values for the H4 (1-21) WT substrate of 0.39 μM, 5.63 h−1, and 2.83, respectively. For H4 (1-21) R17MMA, the parameters were 0.13 μM, 9.25 h−1, and 1.3, respectively. For details about reaction conditions and concentrations, see Methods. Statistical analysis of K0.5 values (C), statistical analysis of kcat values (D), and statistical analysis of the Hill coefficient values (E). The dashed line represents a Hill coefficient of 4. Data were taken from the triplicate assays shown in A and B; error bars represent SD. The P values were calculated using a one-way ANOVA test with a Dunnett test for multiple comparisons using the GraphPad Prism 6.0 software.