Significance
Viable egg production by Schistosoma species is the key pathogenic process causing granuloma formation in permissive hosts (e.g., mice), while nonpermissive hosts [e.g., Norway rats (Rattus norvegicus)] avoid such sequelae. Using inducible nitric oxide synthase knockout (iNOS−/−) rats, we demonstrate that high expression levels of iNOS in rats play an important role in blocking the egg-induced granuloma formation of Schistosoma japonicum. The nitric oxide, produced by iNOS, inhibits parasite growth, reproductive organ development, egg production, and viability by interfering with mitochondrial function. This study solves the puzzle as to why rats are naturally resistant to S. japonicum infection and provides insights for understanding the pathogenesis of human schistosomiasis and the interactions between host and parasite.
Keywords: rat, Schistosoma japonicum, schistosomiasis, granuloma formation, mitochondria
Abstract
Human schistosomiasis, caused by Schistosoma species, is a major public health problem affecting more than 700 million people in 78 countries, with over 40 mammalian host reservoir species complicating the transmission ecosystem. The primary cause of morbidity is considered to be granulomas induced by fertilized eggs of schistosomes in the liver and intestines. Some host species, like rats (Rattus norvegicus), are naturally intolerant to Schistosoma japonicum infection, and do not produce granulomas or pose a threat to transmission, while others, like mice and hamsters, are highly susceptible. The reasons behind these differences are still a mystery. Using inducible nitric oxide synthase knockout (iNOS−/−) Sprague–Dawley rats, we found that inherent high expression levels of iNOS in wild-type (WT) rats play an important role in blocking growth, reproductive organ formation, and egg development in S. japonicum, resulting in production of nonfertilized eggs. Granuloma formation, induced by fertilized eggs in the liver, was considerably exacerbated in the iNOS−/− rats compared with the WT rats. This inhibition by nitric oxide acts by affecting mitochondrial respiration and energy production in the parasite. Our work not only elucidates the innate mechanism that blocks the development and production of fertilized eggs in S. japonicum but also offers insights into a better understanding of host–parasite interactions and drug development strategies against schistosomiasis.
Schistosomiasis, caused by Schistosoma species, is the second most important parasitic disease for public health after malaria. In 2015, it was estimated that 700 million people were at the risk of this disease and 218 million people required treatment in 78 countries (1). Schistosoma japonicum is widely distributed in South China, Indonesia, and the Philippines (1), with 170,438 patients being treated in China in 2015 (2). It is well known that viable egg production is the key for both transmission and pathogenesis (egg-induced granulomas in the liver and intestinal tissues) of this parasite. The female S. japonicum produces around 3,000 eggs per day, 10-fold more than the related species Schistosoma mansoni, and this has been proposed to cause a more severe pathology to patients (3). S. japonicum is one of the most difficult parasites to control, because more than 46 nonhuman mammals can be naturally infected, especially cattle, goats, dogs, pigs, and mice, and these play an important role in the transmission of this disease in endemic regions (4, 5). However, it is well known that some experimental animals, such as Norway rats (Rattus norvegicus), show an innate resistance to infection, in which Schistosoma spp. cannot develop well and do not cause typical granuloma formation in the liver (6–8). These phenomena are described as susceptible or “permissive” and resistant or “nonpermissive” based on the capacity of the host species to allow development of sexual maturation and oviposition by the parasite (7). Although such natural characteristics have been investigated for several decades, little direct evidence has been obtained to fully explain these phenomena, even despite the publication of the genome of S. japonicum and the great benefits provided by it (9). We are interested to know why such huge differences in resistance occur between mice and rats when they are infected with S. japonicum. What are the host factors that relate to innate resistance? Obviously, a better understanding of the mechanism of resistance would provide a better understanding of the pathogenesis of human schistosomiasis and the host–parasite interactions.
In recent decades, experiments have been carried out to investigate potential mechanisms that could mediate natural resistance to Schistosoma infection in rats. Capron and Capron (6) and Capron et al. (10), primarily using in vitro assays, argued that humoral immunity, particularly antibody-dependent, cell-mediated cytotoxicity, played a critical role in the resistance of the rat host. In addition, the anaphylactic antibodies (IgG2a and IgE) and effector cells, including eosinophils, macrophages, platelets, and mast cells, could mediate cytotoxicity, which might act directly against schistosomula in vivo (10, 11). However, some contrasting results have indicated that cell-mediated responses appeared to be insignificant in rat schistosomiasis (12). Moreover, some results also suggested that a Th2 type response was involved in such resistance, based on the observations of preferential expression of Th2 cytokines before rejection of worms in infected rats (12–14). Endocrine gland removal studies revealed that hormones from the pituitary and thyroid/parathyroid glands were required for innate resistance in rats (15), but no specific hormones were identified due to technical limitations at the time. Nevertheless, none of these proposed mechanisms could definitely and satisfactorily fully explain the resistance.
A comparison between mice and rats has clearly shown that the expression levels of inducible nitric oxide synthase (iNOS or NOS2) and the production of nitric oxide (NO) are barely detectable in naive mice but significantly higher in naive rats (16). This production of NO is typically dependent on l-arginine metabolism by iNOS in activated macrophages and other immunocytes in response to microbial compounds and/or cytokines (e.g., IFN-γ, IL-1) (17, 18). NO has been identified as participating in macrophage-mediated killing or cytostasis of various extracellular or intracellular parasitic protozoans, such as Toxoplasma, Leishmania, Plasmodium, and Trypanosoma (17). In fact, some studies on NO have also been carried out on Schistosoma in a mouse model. For example, it was reported that macrophage and endothelial cell-mediated cytotoxicity against schistosomula of S. mansoni in vitro might be involved in the production of NO (19, 20). In addition, Wynn et al. (21) found that worm burdens were increased when mouse NO synthase activity was inhibited by aminoguanidine, a selective inhibitor of iNOS. Nevertheless, all of these studies were based on mouse models, and the role of NO in rats on infection by S. japonicum remains a mystery. We therefore hypothesized that the mechanism of natural resistance/intolerance to S. japonicum infection in rats could be related to inherent high expression levels of iNOS.
To test this hypothesis, iNOS knockout (iNOS−/−) Sprague–Dawley (SD) rats were used. We found that inherent high expression levels of iNOS in wild-type (WT) rats play a key role in blocking S. japonicum growth, reproductive organ development, egg production, and the ability to lay fertilized eggs. The consequences of this were to limit granuloma formation in the liver. We show that this inhibition by NO acts by affecting mitochondrial respiration and energy production in the worm. These findings not only provide direct evidence to demonstrate that NO is the key factor for natural resistance to S. japonicum infection in rats but also provide knowledge for a better understanding of the pathogenesis of schistosomiasis. They also inform potentially novel strategies to design new compounds and drugs to control schistosomiasis.
Results
NO Is a Key Molecule in Rats That Hampers the Development of S. japonicum.
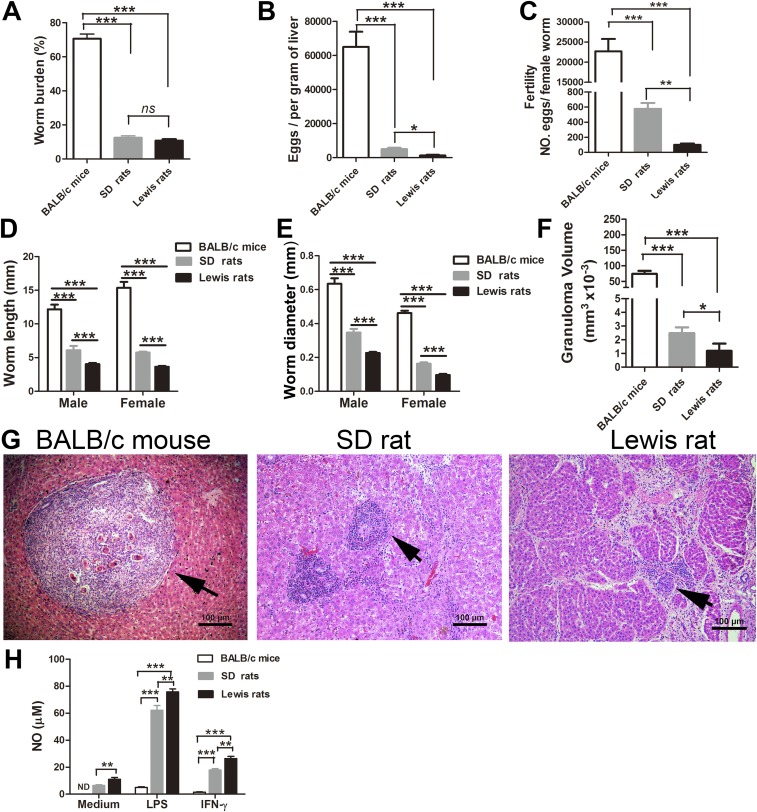
To test the hypothesis that NO plays an important role in the natural resistance/intolerance to S. japonicum infection in rats, initial studies were carried out to compare the status of NO production in BALB/c mice and SD and Lewis rats postinfection with S. japonicum. As expected, based on previous studies, development and fecundity levels of the parasite, parasite loads, and the size of granulomas in the tested animals were negatively correlated with their capacity to produce NO (Fig. S1), implying the inhibitory effect of NO in S. japonicum growth, maturation, fecundity, and pathogenesis.
Fig. S1.
Susceptibility to S. japonicum infection in mice and rats is correlated with their NO production levels in peritoneal macrophages. (A) Parasite burdens in BALB/c mice, SD rats, and Lewis rats after infection with S. japonicum at 7 wk. (B) Trapped eggs in liver were enumerated by microscopy at 7 wk postinfection. (C) Fertility was calculated as numbers of eggs produced per female worm. (D and E) Lengths and diameters of parasites were measured from digital micrographs. (F) Size of liver granulomas at 7 wk postinfection. (G) Representative granulomas at 7 wk postinfection, as indicated by arrows. Liver sections were stained with H&E. (Magnification: 10×.) (Scale bars: 100 μm.) (H) Levels of NO production in peritoneal macrophages from BALB/c mice, SD rats, and Lewis rats after 24 h of stimulation with LPS (100 ng/mL), stimulation with IFN-γ (50 ng/mL), or not stimulated. Data are expressed as the mean ± SEM of five rats or mice per group. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant; ND, not detectable.
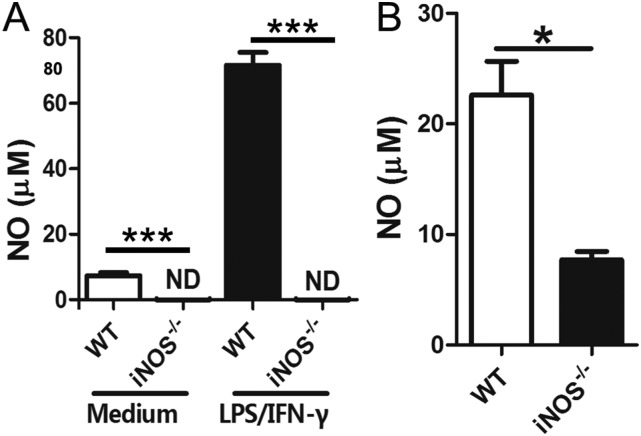
Furthermore, iNOS−/− SD knockout rats were generated with undetectable NO production in peritoneal macrophages and lower levels of NO in sera (Fig. S2). Following infection, iNOS−/− rats showed a significant increase in worm burden (iNOS−/− rat, 82 ± 4; WT rat, 21 ± 2; P < 0.001) and egg deposition in the liver (eggs per gram of liver tissue: iNOS−/− rat, 106,334 ± 19,955; WT rat, 4,903 ± 1,239; P < 0.001; Table 1). Notably, the worm fecundity in the iNOS−/− rats, defined as the average egg production per female, was found to be nearly fivefold higher than that found in WT rats (Table 1).
Fig. S2.
Difference in iNOS expression and NO production in WT and iNOS−/− rats. (A) NO production in peritoneal macrophages from WT rats and iNOS−/− rats after induction with LPS (100 ng/mL) plus IFN-γ (50 ng/mL). Supernatants were collected and analyzed after 24 h of induction. Noninduced cells were maintained in culture medium as controls. ND, not detectable. (B) NO concentration in serum of WT rats and iNOS−/− rats. The data are expressed as the mean ± SEM of five rats per group. *P < 0.05; ***P < 0.001. Data are representative of three independent experiments.
Table 1.
Worm and egg burden in WT compared with iNOS−/− SD rats at 7 wk after S. japonicum infection
| Groups | Total | Male worms | Female worms† | No. of eggs found in liver, per gram | Eggs per female worm (range) |
| WT | 21 ± 1.7 | 12 ± 1.3 | 9 ± 0.8 | 4,903 ± 1,239 | 509 ± 94 (104–660) |
| iNOS−/− | 82 ± 4.2*** | 42 ± 2.5*** | 40 ± 2.3*** | 106,334 ± 19,955*** | 2,922 ± 548** (1,108–4,924) |
WT and iNOS−/− rats were infected percutaneously with 200 cercariae of S. japonicum. Worm and egg burdens were determined at 7 wk postinfection. Data are expressed as the mean ± SEM (n = 10). Significant differences in characteristics were noted between WT and iNOS−/− rats. **P < 0.01; ***P < 0.001.
The female worm numbers also indicate the numbers of pairs, as they were always found in pairs.
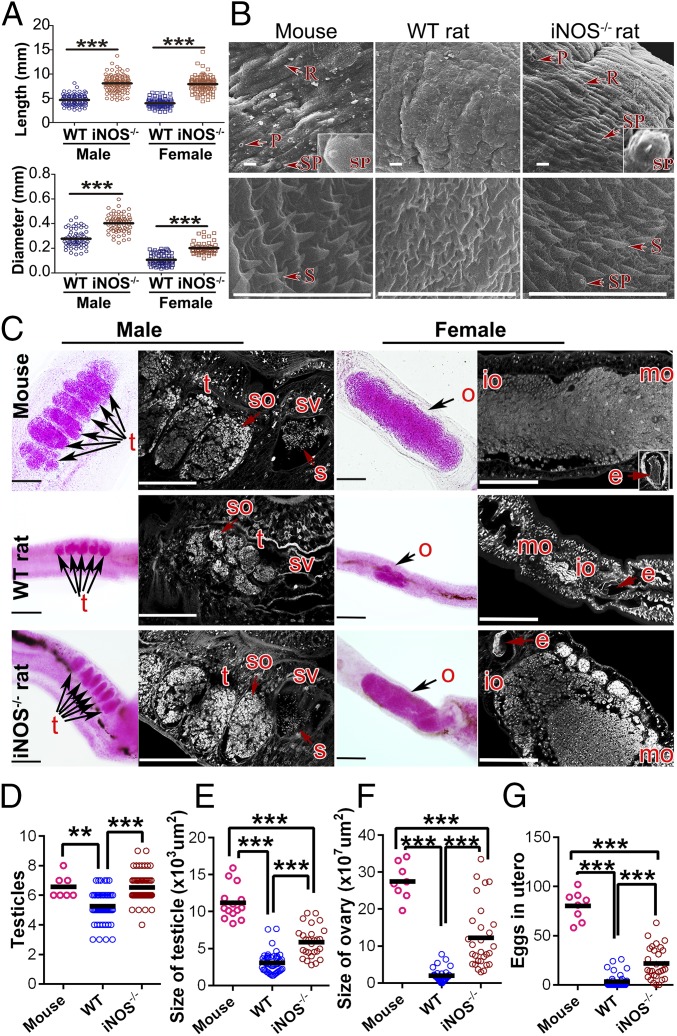
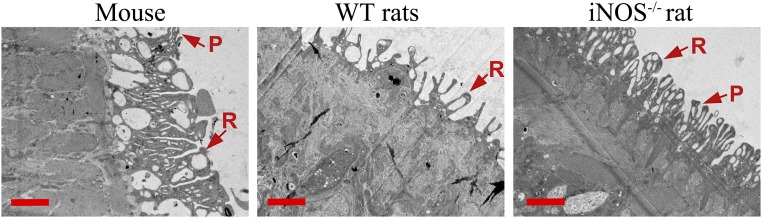
To better understand the effects on S. japonicum in the iNOS−/− rat, detailed biological characteristics of the worms were examined. The lengths and diameters of male and female worms collected from iNOS−/− rats at 7 wk postinfection were significantly greater than those obtained from WT rats (Fig. 1A). The tegument of S. japonicum from the infected iNOS−/− rats was covered with well-arranged ridges and abundant pits, as well as sensory papillae with setae, and was similar to that of worms collected from mice (Fig. 1B and Fig. S3). However, in contrast, these characteristics were poorly developed in the worms from WT rats (Fig. 1B and Fig. S3). In addition, a large number of spines and several sensory papillae were found in the tegument of oral suckers of S. japonicum from iNOS−/− rats and mice but were not observed in the WT rat group (Fig. 1B).
Fig. 1.
Development of adult S. japonicum in WT and iNOS−/− rats. BALB/c mice and WT and iNOS−/− rats were infected with S. japonicum, and parasites were harvested at 7 wk postinfection. (A) Length and diameter of male and female worms were measured from digital micrographs. (B) Scanning electron microscopy (SEM) analysis of the body tegument (Upper) and tegument in the oral sucker (Lower) of adult male worms. R, ridge; P, pit; S, spine; SP, sensory papillae. (Scale bars: 10 μm.) (C) Morphological analysis of reproductive organs of worms. The worms were stained with hydrochloric carmine and observed under a light microscope (Left) and confocal laser scanning microscopy (Right). e, egg; io, immature oocytes; mo, mature oocytes; o, ovary; ot, ootype; s, sperm; so, spermatocytes; sv, seminal vesicle; t, testicular lobules. (Scale bars: 100 μm.) (D–G) Quantitative analysis of data from C. Mean values are represented by horizontal bars. **P < 0.01; ***P < 0.001. Groups of six to 10 rats or mice were used for each experimental condition. Data are representative of at least three independent experiments.
Fig. S3.
Transmission electron microscope analysis of the tegument of adult male worms obtained from a mouse and WT and iNOS−/− rats. P, pit; R, ridge. (Scale bars: 2 μm.)
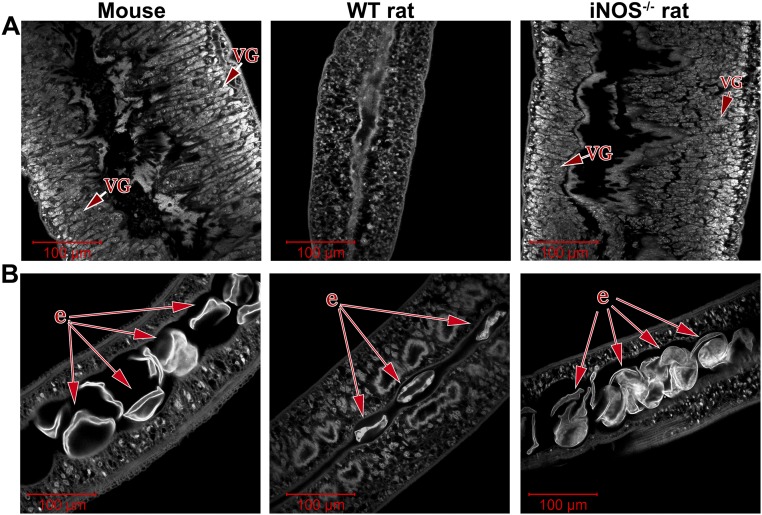
Most importantly, we also found a huge difference between the reproductive systems of S. japonicum collected from iNOS−/− and WT rats. The testes of adult male schistosomes from iNOS−/− rats were composed of six to eight testicular lobes containing large amounts of spermatogonia and spermatocytes, while the seminal vesicle was filled with thousands of mature sperm (Fig. 1C). In the control mice, S. japonicum had a similar phenotype as in the iNOS−/− rats. In contrast, in WT rats, S. japonicum displayed a significant reduction in the number and size of testicular lobes, accompanied by a remarkable decrease in cell density in the testes plus a lack of mature sperm (Fig. 1 C–E). Furthermore, in female worms, drastic differences were observed in the size of ovaries, vitellaria, and numbers of nonexcreted eggs in the uterus of S. japonicum collected from the iNOS−/− and WT rats (Fig. 1 C, F, and G and Fig. S4). Analogous to the worms observed in mice, we found that the ovaries of mature female worms collected from the iNOS−/− rats were composed of abundant oogonia, immature and primary oocytes (Fig. 1 C and F), while the uteri were filled with eggs (Fig. 1G and Fig. S4B) and the vitelline lobes were clustered with closely arranged vitelline cells (Fig. S4A). In contrast, there were significant reductions in the diameters of ovaries that contained only a few oocytes in the female worms collected from the WT rats (Fig. 1 C and F). The occurrence of eggs in uteri was rare, and those present were not properly formed; the vitelline lobes had scantily organized vitelline cells (Fig. 1G and Fig. S4). These results obtained from the WT rats are consistent with those previously described (8), but show a clear difference from phenotypes observed in the iNOS−/− rats.
Fig. S4.
Vitelline glands and the uterus in the female S. japonicum observed using confocal laser scanning microscopy. Worms collected from BALB/c mice and WT and iNOS−/− rats were stained with hydrochloric carmine and observed by confocal laser scanning microscopy. (A) Vitelline glands of female worms. (B) Eggs in the uterus of female worms. e, egg; VG, vitelline glands.
S. japonicum Produced Viable Eggs in the Infected iNOS−/− Rats.
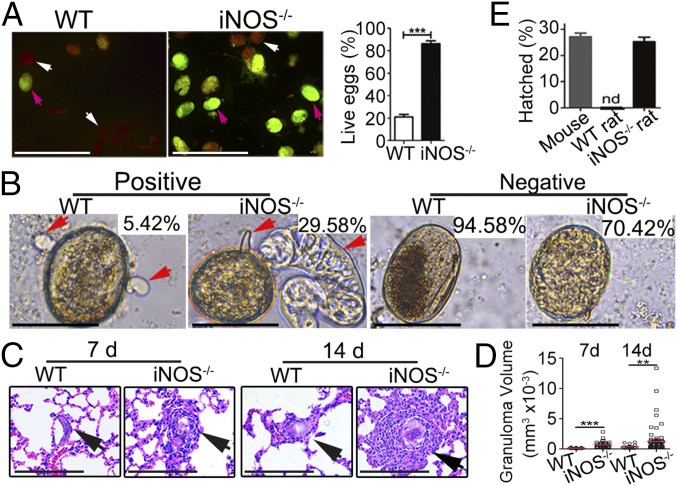
To investigate the hypothesis that S. japonicum should produce nonfertilized eggs and underdeveloped embryos in the WT rats, acridine orange fluorescence staining was used as a detection system to measure viable egg production. We found a lower percentage (21.05%) of live eggs of S. japonicum from the WT rats, compared with a much higher percentage (86.28%) of viable eggs from the iNOS−/− rats (Fig. 2A; P < 0.001). Furthermore, results from the circumoval precipitation reaction (CPR), a specific indicator of the secretion activity of viable mature eggs, showed that a characteristic and dense reaction product surrounded 29.58% of 2,000 eggs collected from the iNOS−/− rats, while only weak CPR activity was observed in 5.42% of 1,200 eggs collected from the WT rats (Fig. 2B). Moreover, we found that much more severe pulmonary granulomas were induced by eggs collected from the livers of iNOS−/− rats than those from the WT rats, when injected i.v. into naive mice (Fig. 2 C and D).
Fig. 2.
Characteristics of S. japonicum eggs collected from WT and iNOS−/− rats. (A) Acridine orange staining of S. japonicum eggs. White arrows indicate the dead eggs, and magenta arrows indicate the live eggs. (Scale bars: 100 μm.) (B) Circumoval precipitation (red arrows) surrounding eggs indicated the secretion activity of live mature eggs. The percentages of positive and negative eggs with the CPR were noted. (Scale bars: 20 μm.) (C) Pulmonary granuloma formation in BALB/c mice induced by schistosome eggs collected from the livers of WT and iNOS−/− rats, respectively. Histological analysis of lungs by H&E staining after 7 and 14 d. (Scale bars: 50 μm.) (D) Size of pulmonary granulomas from C. (E) Hatching of the eggs of S. japonicum. nd, nondetectable. The data are expressed as the mean ± SEM of five animals per group. **P < 0.01; ***P < 0.001. Data are representative of three independent experiments.
To test the developmental status of S. japonicum eggs from the infected iNOS−/− and WT rats, a hatching test was carried out. Parasite eggs recovered from the iNOS−/− rats were capable of hatching to miracidia (25.3%) in a similar proportion to those recovered from mice (Fig. 2E). However, in contrast to the results from the iNOS−/− rats, eggs collected from the WT rats were unable to hatch, thus demonstrating that NO has a specific role in affecting egg viability.
Exacerbated Granuloma Formation in the iNOS−/− Rats Was Attributed Only to the Full Development of Parasites, Not to Other Host Factors.
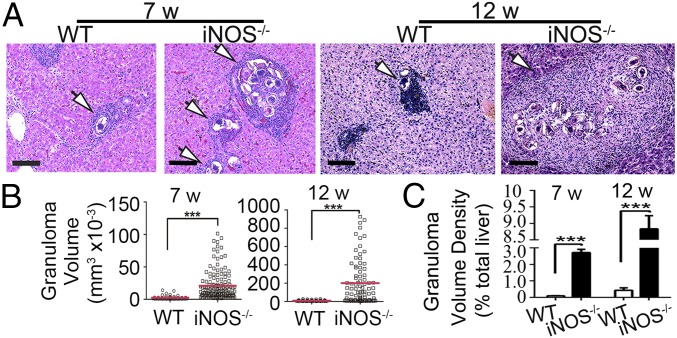
It is well known that viable eggs of schistosomes are a key factor for the formation of granulomas in their hosts (22). Indeed, as we predicted, rare and small granulomas were found in the liver of WT rats infected with S. japonicum at 7 and 12 wk postinfection, while both the number and size of granulomas were dramatically increased in the infected iNOS−/− rats (Fig. 3 A–C). The size of hepatic granulomas in iNOS−/− rats infected with S. japonicum was 20.97 ± 1.87 (×10−3 mm3) at 7 wk postinfection, nearly eightfold larger than those found in the infected WT rats [2.56 ± 0.42 (×10−3 mm3); P < 0.001]. In a follow-up at 12 wk postinfection, the size of hepatic granulomas, remarkably, increased to 201.18 ± 25.91 (×10−3 mm3) in iNOS−/− rats infected with S. japonicum, over 22-fold larger than those found in the infected WT rats [8.79 ± 0.83 (×10−3 mm3); P < 0.001; Fig. 3B]. Furthermore, comparison of the granuloma density of iNOS−/− and WT rats showed an increase of 30-fold and 20-fold, respectively, in liver tissue in the knockout rats at 7 wk (WT: 0.09 ± 0.01%; iNOS−/−: 2.73 ± 0.19%; P < 0.001) and 12 wk (WT: 0.43 ± 0.14%; iNOS−/−: 8.84 ± 0.40%; P < 0.001) postinfection (Fig. 3C). The marked increase in hepatic granulomatous inflammation in the infected iNOS−/− rats was largely dependent on the increased egg production of S. japonicum and maturation of eggs (Table 1 and Fig. 2B).
Fig. 3.
Egg-induced granulomatous inflammation in livers and lungs in WT and iNOS−/− rats. (A) Representative H&E staining images of hepatic granulomas at 7 wk and 12 wk postinfection with 200 S. japonicum cercariae. (Scale bars: 100 μm.) Arrows identify egg-induced granulomas. (B) Size range of liver granulomas (WT groups, n = 49 and n = 69; iNOS−/− groups, n = 138 and n = 90). (C) Granuloma volume density in liver tissue. Granulomas were measured in tissue section (>8.2 mm3) in five individual rats per group. The data are expressed as the mean ± SEM. ***P < 0.001. Data are representative of three independent experiments.
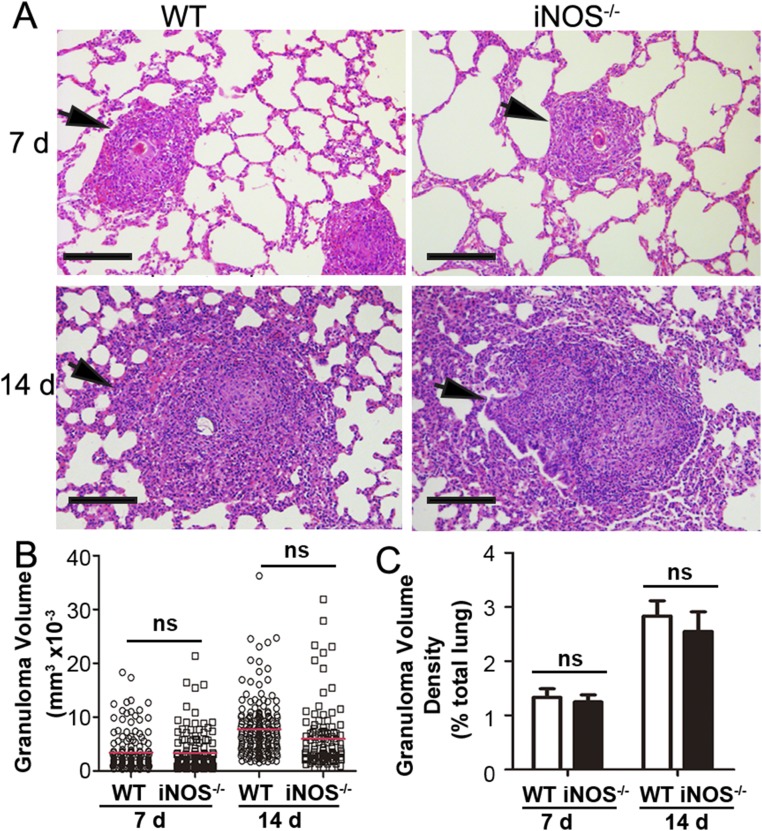
To exclude the possibility that the changes in host immunity factors post-NO deficiency may contribute to the hepatic granuloma, pulmonary granulomas were compared in the WT and iNOS−/− rats after injection of eggs obtained from rabbits infected with S. japonicum. To our surprise, similar sizes and volume density of pulmonary granulomas were observed in both the WT and iNOS−/− rats after injection of the same dose of viable mature eggs (Fig. S5). Thus, our results clearly demonstrated that the exacerbation of hepatic granulomas in the iNOS−/− rats was not attributed to host factors, but to the viability of Schistosoma eggs.
Fig. S5.
Pulmonary granuloma formation in WT and iNOS−/− rats induced by schistosome eggs collected from rabbits. WT and iNOS−/− rats were injected i.v. with 15,000 eggs, and lungs were removed for histological analysis after 7 and 14 d. (A) Representative images of pulmonary granuloma. (Scale bars: 100 μm.) (B) Size of a single granuloma. (C) Granuloma volume density in lung tissue (>7.8 mm3). The data are expressed as the mean ± SEM. Data are representative of three independent experiments. ns, not significant.
Adoptive Transfer of WT Macrophages into the iNOS−/− Rats Could Partially Restore the Inhibition Against S. japonicum.
To provide further evidence of the role of NO on the inhibition of development of S. japonicum, adoptive transfer of WT rat macrophages (Mφ) into iNOS−/− rats was performed. Macrophages were used as they are considered to be the best-characterized source of NO (18). After transfer, the iNOS−/− recipient rats (iNOS−/− + Mφ) that received adoptive transfer of WT macrophages were able to express iNOS (Fig. S6 A and B) and elevated the production of NO in vivo (Fig. S6C). As seen in Table S1, in contrast to the status in the iNOS−/− rats, the worm burden and egg production, together with worm fecundity, were significantly reduced in the recipient group of animals (iNOS−/− + Mφ). Furthermore, we found that the adoptively transferred macrophages could partially inhibit the parasite growth, which resulted in a decrease in length and diameter (Fig. S6 D–F). As a consequence, the size of granulomas in livers displayed a marked reduction in the iNOS−/− + Mφ group (Fig. S6 G and H). Thus, these data further demonstrated that NO is a key factor involved in blocking the development of S. japonicum in rats.
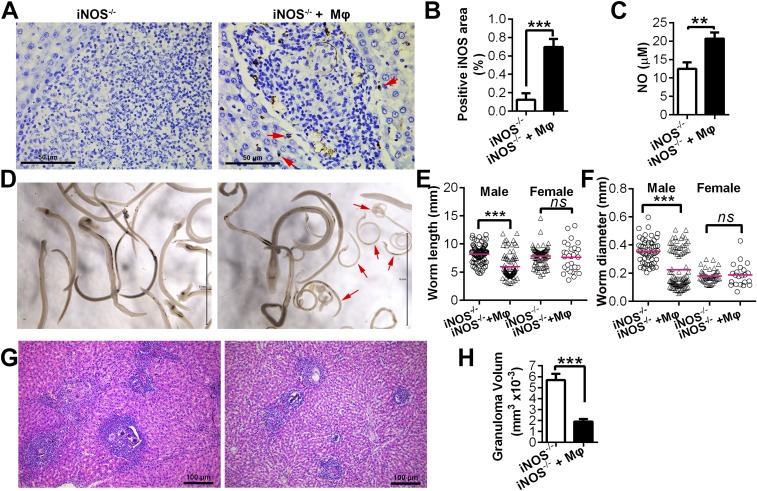
Fig. S6.
Adoptive transfer of WT macrophages into infected iNOS−/− rats. Adoptive transfer of WT macrophages was performed in iNOS−/− rats, as described in SI Materials and Methods (group iNOS−/− + Mφ), simultaneously with a group of infected iNOS−/− rats that did not receive macrophages; instead, PBS was used as an additional control (group iNOS−/−). The rats were killed on day 43 (6 wk postinfection). (A) Expression of iNOS in liver was identified by immunohistochemistry using an iNOS antibody. Arrows indicate the iNOS signal. (B) Quantitation of the positive area of fields of view showing iNOS expression. (C) NO concentration in the serum of infected iNOS−/− rats and iNOS−/− recipients at 6 wk postinfection. (D) Representative micrographs showing parasites present. Arrows identify stunted parasites. (E and F) Worm lengths and diameters were measured from digital micrographs. Mean values are represented by horizontal bars. (G) H & E stain of representative hepatic granulomas. (H) Quantitation of hepatic granuloma sizes as measured from H&E-stained slides. Data are expressed as the mean ± SEM from two biological repeats (n = 10). **P < 0.01; ***P < 0.001. ns, not significant. Scale bars represent 50 μm (A), 5 mm (D), or 100 μm (G), respectively.
Table S1.
Worm and egg burden in iNOS−/− rats without macrophage transfer compared with macrophage transfer (iNOS−/− + Mφ) of iNOS−/− rats at 6 wk after S. japonicum infection
| Groups | Total | Male | Female | No. of eggs found in liver, per gram | Eggs/female worm |
| iNOS−/− | 106 ± 5 | 58 ± 3.5 | 49 ± 1.5 | 18,568 ± 3,158 | 379 ± 60 |
| iNOS−/− + Mφ | 67 ± 5.5* | 50 ± 2.2 | 17 ± 3** | 3,034 ± 354** | 176 ± 20* |
The iNOS−/− rats were infected percutaneously with 200 S. japonicum cercariae. Macrophages from WT rats were transferred into iNOS−/− recipients as described in SI Materials and Methods. Data are expressed as the mean ± SEM from two biological repeats (n = 10). *P < 0.05; **P < 0.01.
NO Inhibits the Mitochondrial Respiration of S. japonicum.
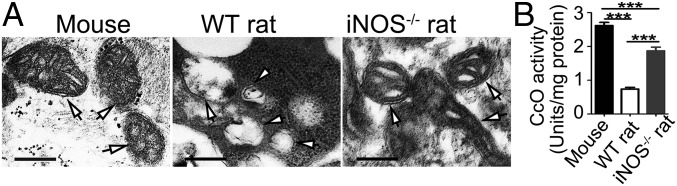
In this study, we speculated that the mechanisms of NO blocking the development of Schistosoma might be linked to the inhibition of mitochondrial respiration, resulting in inhibition of mitochondrial energy production and lethal metabolic interference. To test this hypothesis, the mitochondrial morphology and structure of S. japonicum were compared. Ultrastructural observations revealed that worms from the mice had typical eukaryotic mitochondria with well-defined outer membranes and a clear cristae structure. In contrast, clusters of damaged mitochondria exhibiting mitochondrial swelling and distortion, loss of intact internal membranes, and disruption of mitochondrial cristae with vacuolization were observed in worms from the WT rats. However, mitochondrial alterations were considerably diminished in the worms from the iNOS−/− rats (Fig. 4A and Fig. S7). In addition, the relative mRNA expression of the mitochondrial respiratory chain enzymes, cytochrome c oxidase (CcO, complex IV) subunit I and NADH dehydrogenase (complex I), in worms from the WT rats was significantly decreased (Fig. S8). CcO activity was also significantly decreased in worms from the WT rats, compared with those from the iNOS−/− rats and mice (Fig. 4B). These results strongly suggest that the mechanisms of NO blocking the development of S. japonicum in rats act by affecting mitochondrial respiration in the parasite.
Fig. 4.
Mitochondrial respiration was inhibited in worms collected from WT rats. S. japonicum was harvested from infected animals at 7 wk postinfection. (A) Ultrastructural analysis of mitochondria in worms. Arrows indicate mitochondria. (Scale bars: 200 nm.) (B) Respiratory chain enzyme CcO activity from isolated mitochondria of adult worms. The data are expressed as the mean ± SEM. ***P < 0.001. Data are representative of three independent experiments.
Fig. S7.
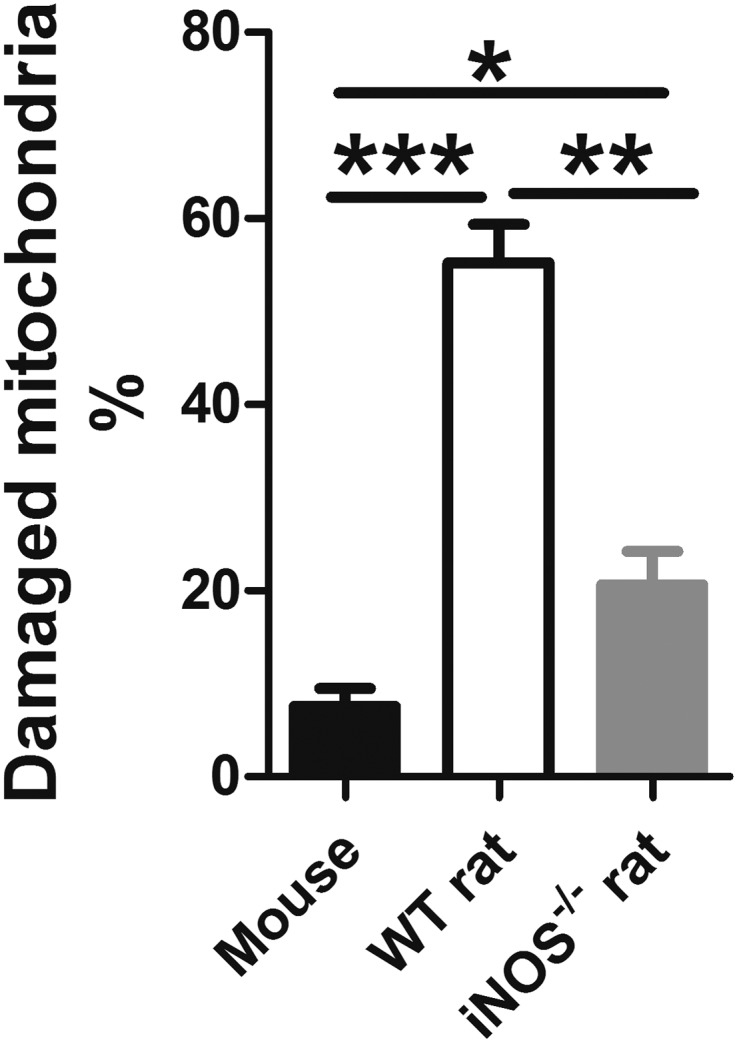
Damaged mitochondria from testicular tissue and ovarian cross-sections were counted using a transmission electron microscope, including 342 mitochondrial organelles from four worms from the mouse group, 254 mitochondrial organelles from four worms from the WT rat group, and 309 mitochondrial organelles from four worms from the iNOS−/− rat group. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. S8.
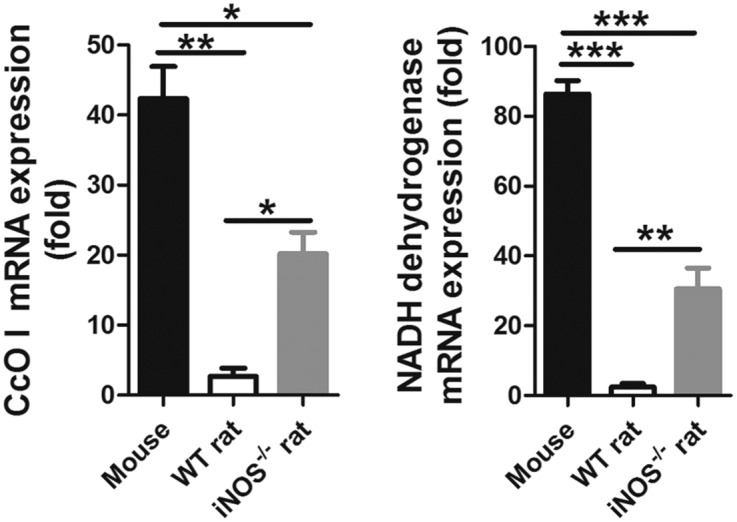
Relative expression of the mitochondrial respiratory chain enzyme CcO I and NADH dehydrogenase in WT and iNOS−/− rats. The relative expression of each gene was normalized to the expression of β-actin. Data are shown as the mean ± SEM from two biological repeats (n = 10). Significant differences have been noted. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Understanding defense mechanisms against parasites is a key aspect of elucidating host–parasite interactions. S. japonicum is a zoonotic parasite with a naturally wide permissive host range; however, some hosts, including the brown rat, are nonpermissive hosts. This provides a good model system for investigating the host–parasite interactions that control and limit infection. In permissive hosts, such as mice and hamsters, the parasites are able to reach sexual maturation and deposit eggs, which then trigger the formation of granulomas that are ultimately responsible for mortality. However, in nonpermissive hosts, such as rats, the parasites struggle to survive and do not fully develop into mature stages (7, 8, 15). Scientists have long been puzzled by these biological differences among mammalian species, and the causative mechanism(s) remained unclear; many hypotheses have been proposed to account for this (6, 12, 14).
In early studies based on the mouse model, evidence indicated the effect of NO on killing Schistosoma (17), but the mechanism was not clarified. Based on our results from the rat models (WT vs. iNOS−/− and adoptive transfer of macrophages), we have demonstrated that high expression of iNOS with a higher amount of NO in rats is strongly linked to the inhibition of development of S. japonicum, and is a key factor contributing to their resistance against the parasite. The huge differences in development of S. japonicum between the WT and iNOS−/− rats clearly showed that NO could significantly influence the tegument structures, body size, and development of the reproductive organs in S. japonicum. The tegument is known to be required as essential protection for parasite survival during host immune attacks (23) and as a key structure for driving nutrient absorption and cholesterol metabolism (24, 25). The modified structure of the tegument of S. japonicum in WT rats causes significant problems for the absorption of nutrients and the development of the parasite. Importantly, we found that the reproductive organs of the female worms were not properly formed in the infected WT rats, represented as significant decreases in the size of ovaries and the number of vitelline cells and nonexcreted eggs compared with those found in iNOS−/− rats and mice groups. These deformities led to a significant decrease in both egg production and deposition in the tissues of the host. This, in turn, alleviated the pathogenesis caused by egg deposition. In fact, early evidence obtained from the mouse model system indicated similar effects of NO on S. mansoni when NO production was elevated by chemical compounds (26) or vaccination (27). Interestingly, the inhibition of development and fecundity by NO was also found in Cooperia oncophora, a parasitic nematode in cattle, in which elevated expression of iNOS was observed in acquired resistance during reinfection of this parasite (28). Perhaps this represents a generic effect of NO in helminths. Alongside effects in females, we also found that NO could cause notable reductions in testicular lobe formation (both in size and quantity) and lack of production of mature sperm in the males of S. japonicum. In WT rats, the most significant effect of NO on the inhibition of S. japonicum was the production of nonfertilized eggs. This was manifested as a significant decrease in the proportion of viable eggs, showing a weak CPR and inability to lead to hatching of the important miracidial stages that are required for transmission to new hosts. Interestingly, removal of the pituitary gland and thyroid/parathyroid glands from rats before infection with S. mansoni resulted in increasing worm burdens, worm development, oviposition, and miracidial development (15). Indeed, growth hormone and thyroid hormones have been demonstrated to directly induce iNOS expression and increase iNOS activity by influencing the maturation and function of immune cells, such as macrophages (29–33). These results strongly support the important role of NO in the development of Schistosoma.
Egg granuloma formation in the liver and intestinal tissues of many permissive mammalian hosts, such as mice, has long been considered to be the primary cause of morbidity of schistosomiasis (34). This is reported to be caused by antigens secreted by the mature viable eggs (22), followed by induction of inflammatory cells surrounding the eggs (34). In fact, rare egg granulomas have also reportedly been found in nonpermissive hosts (7, 8). In our work, we found that S. japonicum worms developing in WT rats laid 20-fold fewer eggs than those developing in the iNOS−/− rats. Surprisingly, the magnitude of change in egg granuloma production (volume density) between WT and iNOS−/− rats was more than 30-fold. This clearly indicated that the viability of eggs contributed to the difference observed. This result is consistent with the traditional concept that only viable eggs of schistosomes are able to induce granuloma formation in their hosts (22). However, it was still unclear in previous studies why such obvious differences occur between permissive and nonpermissive hosts during infection with S. japonicum. This was largely attributed to host specificity, although detailed mechanisms were not then forthcoming.
There was some evidence suggesting that NO could play a direct role in limiting granulomatous inflammation in iNOS−/− mice infected with S. mansoni (35) and in the in vitro granuloma reaction with the iNOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) (36). However, this inhibition effect was not observed when aminoguanidine was administered to mice infected with S. mansoni (37), and it was not observed when the inhibitors l-N6-(iminoethyl)-lysine and l-NAME were used in a model where hepatic granulomas were induced by implanting S. japonicum eggs (38); however, the toxicity of these inhibitors to the parasites had not been clarified. In our study, we were able to exclude the effect of host immunity factors, post-NO deficiency, on egg granuloma formation and attribute it solely to the quantity and viability of parasite eggs. This was confirmed by observing a similar volume density and size of pulmonary granulomas formed in both the WT and iNOS−/− rats following injection with the same dose of viable mature eggs.
NO is an unusual effector molecule because of its ability to diffuse freely across cell membranes. This allows it to diffuse into the worm (39) and to directly influence its physiology (e.g., toxic peroxynitrite anion) (17, 40) or to indirectly (e.g., via S-nitrosylation) target inactivation and degradation of iron-containing enzymes (17, 40, 41), which were essential for parasite metabolism (42, 43). For example, earlier studies suggested that NO/nitrite could mediate in vitro schistosomula killing by causing mitochondrial lesions and inhibition of mitochondrial respiration (19, 44). In fact, mitochondrial metabolism, especially the tricarboxylic acid cycle, has been shown to have an essential function in S. japonicum (43). This was demonstrated by using fluoroacetate, an inhibitor of aconitase, and showing that it could cause a separation and hepatic shift of paired worms; a significant fall in both glycogen and protein content; and consequently, a considerable loss of worm body weight. Other studies demonstrated that the mitochondrial respiratory chain also plays an important role in egg production (45) and biosynthetic processes in Schistosoma (46), which are important in rebuilding the surface membrane complex to protect schistosomes from immune attack (23, 46). In addition to the detailed knowledge in Schistosoma, mitochondrial respiration is known to be required for development in other parasitic nematodes (47), suggesting a general role in helminth survival and transmission. Our data clearly show damaged mitochondria in the surviving S. japonicum worms collected from infected WT rats, while observing typical normal mitochondrial structures in the worms collected from the iNOS−/− rats infected with the same parasite. Respiration chain impairment was confirmed by significant decreases in expression levels of complexes I and IV (CcO) and CcO activity, which usually is responsible for 90% of oxygen consumption (48, 49). A large number of pioneering studies have documented that NO can inhibit CcO in competition with oxygen (48, 50, 51). Such suppression of CcO activity is reversible (48, 52). In fact, a parasite transfer study that was carried out in rats showed results consistent with this notion of reversible inhibition of the development and fecundity of Schistosoma (7). By analysis of the function of mitochondrial of S. japonicum collected from the WT and iNOS−/− rats, our results strongly suggest that the mechanisms of NO blocking of the development of S. japonicum in rats act by affecting the mitochondrial respiration.
Taken together, our results demonstrate unequivocally that the key role of NO in blocking the development of S. japonicum may be strongly linked to the inhibition of parasite mitochondrial respiration, which, in turn, leads to decreases in worm survival, egg production, and quantity of fertilized eggs. This consequently limits granuloma formation in the liver and subsequent pathogenesis. By studying the reproductive biology of schistosomes in this way, our results not only solve the long-term puzzle as to why rats are naturally resistant/intolerant to S. japonicum infection but also offer insights into possible control. The knowledge that the interaction and evolution of host and parasite are functionally driven by host NO production suggests new strategies for the design of new compounds and drugs for the control and prevention of human schistosomiasis. We also propose that this iNOS−/− rat model will be a highly beneficial and generic model for determining the role of NO in resistance/intolerance to other pathogen infections.
Materials and Methods
Animals.
Six- to eight-week-old male Bagg albino (BALB/c) mice and SD rats were purchased from the Laboratory Animal Center of Sun Yat-Sen University. Six- to eight-week-old male Lewis rats were purchased from Vital River Laboratories. The iNOS-deficient rats were generated by transcription activator-like effector nucleases (TALENs) technology and breeding at the specific-pathogen-free (SPF) house of Sun Yat-Sen University. The mutant rats are viable and fertile, and do not display any obvious appearance or physical abnormalities. All animals were housed under specific pathogen-free conditions, and this work was approved by the Laboratory Animal Use and Care Committee of Sun Yat-Sen University under license no. 2012CB53000.
Parasite infection.
Cercariae of S. japonicum (Chinese mainland strain) were obtained from infected Oncomelania hupensis snails purchased from the Jiangsu Institute of Parasitic Diseases. Each rat or mouse was percutaneously infected with 200 or 20 cercariae, respectively. Parasites were harvested by perfusion from the portal system.
Other methods used in this paper can be found in SI Materials and Methods.
SI Materials and Methods
Determination of Worm Burdens and Egg Burdens.
Parasites were harvested by perfusion from the portal system of infected animals at 7 wk postinfection. Male and female worms were counted and photographed under a stereoscopic microscope (M205FA; Leica) after separation of paired worms. The length and diameter of worms were measured from digital micrographs using the LAS imaging program (Leica). For this purpose, the male cross-sections of the crescent-shaped (gynecophoral canal) were treated as hollow cylinders.
Egg burdens in tissues were determined as described previously (7). Briefly, liver tissues from the infected animals were weighed and completely digested overnight with 4% potassium hydroxide at 37 °C on a rocking platform. Released eggs were counted under a dissecting microscope.
Macrophages, Isolation, and NO Analysis.
Peritoneal macrophages were isolated as previously described (16). Briefly, rats and mice were killed by CO2 asphyxiation and injected intraperitoneally with 15 mL (rat) or 5 mL (mouse) of ice-cold PBS. The injected PBS with peritoneal cavity fluid was recovered by a plastic syringe and transferred to a sterile centrifuge tube. The harvested suspended cells were centrifuged at 250 × g for 10 min at 4 °C, and the cells were resuspended in RPMI 1640 medium (GIBCO) with penicillin (100 U/mL) and streptomycin (100 mg/mL). Cells were counted and seeded into 24-well culture plates (5 × 105 cells per well) for 2 h at 37 °C with 5% CO2. Then, the wells were washed with FBS-free RPMI 1640 three times to remove nonadherent cells, and fresh RPMI 1640 medium supplemented with 10% FBS (GIBCO), penicillin (100 U/mL), and streptomycin (100 mg/mL) was added. Macrophages were stimulated with LPS (100 ng/mL; Sigma–Aldrich), IFN-γ (50 ng/mL; Sigma–Aldrich), or medium alone. Supernatants were collected 24 h posttreatment, and NO was determined using the Griess reagent as previously described elsewhere (16). Briefly, 100-μL samples were mixed 1:1 with Griess reagent, and absorbance was detected at 550 nm using an ELISA reader (Multiskan MK3; Thermofisher Scientific). Sodium nitrite was used as a standard. NO in serum from animals was determined as described above.
Scanning Electron Microscopy.
Adult worms isolated from BALB/c mice and WT and iNOS−/− rats were fixed individually with 0.2 M PBS containing 2.5% glutaraldehyde (pH 7.4) at 4 °C for 24 h. The samples were washed three times with PBS and six times with distilled water before being dehydrated in gradient ethanol. Following ethanol exchange with acetone and isoamyl acetate, the samples were critical point-dried and then coated with gold in an ion coater (E-102; Hitachi). Worms were observed and photographed using a scanning electron microscope (S-2500; Hitachi).
Transmission Electron Microscopy Analysis.
The samples were fixed, washed, and dehydrated as described above, and then embedded in araldite. Ultrathin sections were cut and contrasted with 1% methanolic uranyl acetate and Reynold’s solution of lead citrate. The sections were observed under a Hitachi H-300 transmission electron microscope.
Reproductive Organ Examination.
The worms were fixed in 95% ethanol, 3% formalin, and 2% glacial acetic acid and stained with 2.5% hydrochloric carmine red (Merck) for 1 h, and then destained in 70% acidic ethanol. Following dehydration in an ethanol gradient, worms were clarified in methyl salicylate and preserved as whole mounts on glass slides. Confocal laser scanning microscopy images were taken using a Zeiss7 DUO NLO microscope with a 488-nm laser and a 470-nm long-pass filter under reflection mode.
Isolation of Eggs.
Schistosoma japonicum eggs were isolated from liver tissues of infected rabbits, mice, and WT and iNOS−/− rats at 45 d postinfection, respectively. After homogenization of the livers in 1.2% NaCl solution, the eggs were collected with a sedimentation glass and were then centrifuged at 1,500 × g for 20 min on Percoll with a density of 1.070 (rabbits and mice) or 1.043–1.056 (rats). The pelleted eggs were stored in sterile 0.9% NaCl solution at 4 °C until use.
Acridine Orange Staining.
The method was followed as previously described (53, 54). Briefly, the purified eggs were mixed with 0.01% acridine orange in an Eppendorf tube and incubated for 2 h at 37 °C. After washing with PBS, a 5-μL aliquot of suspension was placed on a slide and observed under a fluorescence microscope with a 515-nm long-pass reading filter. Live eggs presented as a green and/or red fluorescence showing abundant DNA and/or RNA, while dead eggs exhibited poor staining with only a slight autofluorescence.
Circumoval Precipitation.
A total of 10 μL of egg suspension containing 50–100 eggs was pipetted onto a slide, and one drop of anti-S. japonicum rabbit serum was added. After sealing with a petroleum jelly-bordered coverslip, slides were incubated for 24 h at 37 °C, and results were observed and recorded under a microscope.
Hatching Test.
Eggs were transferred to distilled water and distributed into 96-well culture plates. After counting the number of eggs in each well, the 96-well culture plates were placed under a lamp at room temperature and monitored for the hatching of miracidia in the first 2 h with a dissecting microscope.
Induction of Pulmonary Granulomas.
The induction of pulmonary granulomas was performed as previously described (55). Briefly, S. japonicum eggs were isolated and purified from the livers of infected animals, including rabbits, WT rats, and iNOS−/− rats. A total of 2,000 and 15,000 eggs were injected through the tail vein into mice and rats, respectively. Animals were killed on days 7 and 14 postinoculation, and the left lung was removed for histological analysis.
Histopathology.
Liver and lung tissues were fixed in 4% neutral buffered formalin, embedded in paraffin. Sections were dewaxed and stained with H&E for granuloma analysis. The size of granulomas was calculated as previously described (56). Granuloma volume density, defined as the volume of liver occupied by egg granulomas (57), was quantified by point counting stereology on tissue sections (57, 58).
Adoptive Transfer Experiments.
In this work, 8- to 10-wk-old males of WT SD rats were injected with 3 mL of 2% sterile starch solution (Sigma–Aldrich), and 4 d later, peritoneal macrophages were harvested as described above. A total of 1 × 108 cells suspended in PBS were transferred into iNOS−/− rats through the tail vein on days 0 (before infection), 7, 14, 21, 28, and 35 postinfection with S. japonicum (iNOS−/− + Mφ). A group of NOS−/− rats that received only PBS was used as a control (iNOS−/−). The rats were killed on day 43 (6 wk postinfection) to investigate the status of parasite development.
Immunohistochemistry.
Before immunostaining, liver sections were boiled in 10 mmol/L of citrate buffer for 20 min in a microwave oven for epitope retrieval. After slow cooling and washing with PBS, sections were treated with 3% hydrogen peroxide for 5 min and incubated with 1:100 diluted anti-iNOS antibody (Abcam) overnight at 4 °C. Incubation with a secondary antibody and visualization were done using an UltraVision Quanto Detection System HRP DAB Kit (Thermofisher Scientific). Sections were counterstained with hematoxylin and examined under a microscope.
Assay for CcO Activity.
CcO activity in isolated mitochondria was determined using the Cytochome c Oxidase Assay Kit (Sigma–Aldrich). Protein concentration was determined using the Pierce BCA Protein Assay Kit.
Determination of Mitochondrial Gene Expression by Real-Time PCR.
After perfusion from infected animals, the harvested worms were immediately placed in 0.5 mL of TRIzol (Invitrogen) and mashed using a TissueLyser II (Qiagen). Total RNA was isolated and further purified using an RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. Purified RNA was quantified using a NanoDrop ND-1000 spectrophotometer. First-strand cDNA was synthesized using isolated RNA, SuperScript II reverse transcriptase (Invitrogen), and oligo dT as a primer. Mitochondrial CcO subunit I (CcO I) (GenBank accession no. FN314248.1) and NADH dehydrogenase (GenBank accession no. FN317713.1) were analyzed by qRT-PCR using a LightCycler480 real-time PCR system (Roche, Switzerland) and SYBR green qPCR Master Mixes (Roche). Expression levels of S. japonicum β-actin (GenBank accession no. AF223400.1) were used as endogenous controls within each sample. β-actin primers were as follows: forward, 5′-AGCGTGGTTACAGCTTCACG-3′ and reverse, 5′-AACGCCTCAGGACAACGGAA-3′. CcO I primers were as follows: forward, 5′-TGGGTTCTATTGTGTGTTTGGG-3′ and reverse, 5′-CACGCAACCCACTACTCCCT-3′. NADH dehydrogenase primers were as follows: forward, 5′-TCTGGAAGCCGCACTTGTTG -3′ and reverse 5′-CGAACCGTCAACAGCAAAGGT-3′. Relative expression was calculated using the 2−ΔΔCT method.
Statistical Analysis.
All statistical analyses were performed using SPSS 19.0 software. Significant differences between two groups were determined using a Student’s unpaired t test with Welch’s correction or one-way ANOVA. All data shown represent the mean ± SEM, and P values ≤0.05 were considered statistically significant. At least three to six animals were used per experimental group, and all experiments were performed at least twice.
Acknowledgments
We thank the members of our laboratories, who provided great help during the work. This work was supported by the National Key R&D Program of China (No. 2016YFC1200500) and National Science Foundation of China (Grants 31472058, 31402029, 31672276, and 81572014).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708578114/-/DCSupplemental.
References
- 1.WHO 2016 Schistosomiasis. Available at ( www.who.int/mediacentre/factsheets/fs115/en/). Accessed August 23, 2017.
- 2.Zhang L, et al. Endemic status of schistosomiasis in People’s Republic of China in 2015. Chin J Schisto Control. 2016;28:611–617. doi: 10.16250/j.32.1374.2016246. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LS, Janovy J. Foundations of Parasitology. 7th Ed McGraw–Hill; Boston: 2005. [Google Scholar]
- 4.He YX, Salafsky B, Ramaswamy K. Host–parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17:320–324. doi: 10.1016/s1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- 5.Minggang C, Zheng F. Schistosomiasis control in China. Parasitol Int. 1999;48:11–19. doi: 10.1016/s1383-5769(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 6.Capron M, Capron A. Rats, mice and men - models for immune effector mechanisms against schistosomiasis. Parasitol Today. 1986;2:69–75. doi: 10.1016/0169-4758(86)90158-4. [DOI] [PubMed] [Google Scholar]
- 7.Cioli D, Knopf PM, Senft AW. A study of Schistosoma mansoni transferred into permissive and nonpermissive hosts. Int J Parasitol. 1977;7:293–297. doi: 10.1016/0020-7519(77)90038-8. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, et al. Immune changes of Schistosoma japonicum infections in various rodent disease models. Exp Parasitol. 2012;131:180–189. doi: 10.1016/j.exppara.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capron M, Nogueira-Queiroz JA, Papin JP, Capron A. Interactions between eosinophils and antibodies: In vivo protective role against rat schistosomiasis. Cell Immunol. 1984;83:60–72. doi: 10.1016/0008-8749(84)90225-9. [DOI] [PubMed] [Google Scholar]
- 11.Capron M, et al. Immunologic response of athymic rats to Schistosoma mansoni infection. II. Antibody-dependent mechanisms of resistance. J Immunol. 1983;131:1475–1480. [PubMed] [Google Scholar]
- 12.Khalife J, Cêtre C, Pierrot C, Capron M. Mechanisms of resistance to S. mansoni infection: The rat model. Parasitol Int. 2000;49:339–345. doi: 10.1016/s1383-5769(00)00059-3. [DOI] [PubMed] [Google Scholar]
- 13.Cêtre C, et al. In vivo expression of cytokine mRNA in rats infected with Schistosoma mansoni. Parasite Immunol. 1998;20:135–142. doi: 10.1046/j.1365-3024.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 14.Cêtre C, et al. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun. 1999;67:2713–2719. doi: 10.1128/iai.67.6.2713-2719.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopf PM, Soliman M. Effects of host endocrine gland removal on the permissive status of laboratory rodents to infection by Schistosoma mansoni. Int J Parasitol. 1980;10:197–204. doi: 10.1016/0020-7519(80)90049-1. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, et al. Differences in iNOS and arginase expression and activity in the macrophages of rats are responsible for the resistance against T. gondii infection. PLoS One. 2012;7:e35834. doi: 10.1371/journal.pone.0035834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oswald IP, James SL. Nitrogen oxide in host defense against parasites. Methods. 1996;10:8–14. doi: 10.1006/meth.1996.0072. [DOI] [PubMed] [Google Scholar]
- 18.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 19.James SL, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 20.Oswald IPEI, et al. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci USA. 1994;91:999–1003. doi: 10.1073/pnas.91.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynn TA, et al. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni. J Immunol. 1994;153:5200–5209. [PubMed] [Google Scholar]
- 22.Hang LM, Warren KS, Boros DL. Schistosoma mansoni: Antigenic secretions and the etiology of egg granulomas in mice. Exp Parasitol. 1974;35:288–298. doi: 10.1016/0014-4894(74)90035-6. [DOI] [PubMed] [Google Scholar]
- 23.Abath FG, Werkhauser RC. The tegument of Schistosoma mansoni: Functional and immunological features. Parasite Immunol. 1996;18:15–20. doi: 10.1046/j.1365-3024.1996.d01-6.x. [DOI] [PubMed] [Google Scholar]
- 24.Faghiri Z, Skelly PJ. The role of tegumental aquaporin from the human parasitic worm, Schistosoma mansoni, in osmoregulation and drug uptake. FASEB J. 2009;23:2780–2789. doi: 10.1096/fj.09-130757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira AS, Padilha RJ, Lima-Filho JL, Chaves ME. Scanning electron microscopy of the human low-density lipoprotein interaction with the tegument of Schistosoma mansoni. Parasitol Res. 2011;109:1395–1402. doi: 10.1007/s00436-011-2386-4. [DOI] [PubMed] [Google Scholar]
- 26.Sayed AA, et al. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat Med. 2008;14:407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, et al. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect Immun. 2001;69:386–391. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li RW, Li C, Gasbarre LC. The vitamin D receptor and inducible nitric oxide synthase associated pathways in acquired resistance to Cooperia oncophora infection in cattle. Vet Res (Faisalabad) 2011;42:48. doi: 10.1186/1297-9716-42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P, Menon R. New insights into growth hormone’s actions on the macrophage: Implications for non-growth-related actions of growth hormone. OA Biochemistry. 2013;1:1–10. [Google Scholar]
- 30.De Vito P, et al. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid. 2011;21:879–890. doi: 10.1089/thy.2010.0429. [DOI] [PubMed] [Google Scholar]
- 31.Fernández V, Tapia G, Varela P, Videla LA. Redox regulation of thyroid hormone-induced Kupffer cell-dependent IkappaB-alpha phosphorylation in relation to inducible nitric oxide synthase expression. Free Radic Res. 2005;39:411–418. doi: 10.1080/10715760400029637. [DOI] [PubMed] [Google Scholar]
- 32.van der Spek AH, Fliers E, Boelen A. Thyroid hormone metabolism in innate immune cells. J Endocrinol. 2017;232:R67–R81. doi: 10.1530/JOE-16-0462. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, et al. Thyroid hormone enhances nitric oxide-mediated bacterial clearance and promotes survival after meningococcal infection. PLoS One. 2012;7:e41445. doi: 10.1371/journal.pone.0041445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 35.Hesse M, Cheever AW, Jankovic D, Wynn TA. NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol. 2000;157:945–955. doi: 10.1016/S0002-9440(10)64607-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira DM, Silva-Teixeira DN, Carmo SA, Goes AM. Role of nitric oxide on human schistosomiasis mansoni: Upregulation of in vitro granuloma formation by N omega-nitro-L-arginine methyl ester. Nitric Oxide. 1998;2:57–65. doi: 10.1006/niox.1997.0164. [DOI] [PubMed] [Google Scholar]
- 37.Brunet LR, Beall M, Dunne DW, Pearce EJ. Nitric oxide and the Th2 response combine to prevent severe hepatic damage during Schistosoma mansoni infection. J Immunol. 1999;163:4976–4984. [PubMed] [Google Scholar]
- 38.Hirata M, et al. Effect of nitric oxide synthase inhibition on Schistosoma japonicum egg-induced granuloma formation in the mouse liver. Parasite Immunol. 2001;23:281–289. doi: 10.1046/j.1365-3024.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 39.Gusarov I, et al. Bacterial nitric oxide extends the lifespan of C. elegans. Cell. 2013;152:818–830. doi: 10.1016/j.cell.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 40.Thomas DD, et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stadler J, et al. Inhibition of cytochromes P4501A by nitric oxide. Proc Natl Acad Sci USA. 1994;91:3559–3563. doi: 10.1073/pnas.91.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziniel PD, et al. The Schistosoma mansoni cytochrome P450 (CYP3050A1) is essential for worm survival and egg development. PLoS Negl Trop Dis. 2015;9:e0004279. doi: 10.1371/journal.pntd.0004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang TY. The energy metabolism of Schistosoma japonicum. Int J Biochem. 1980;12:457–464. doi: 10.1016/0020-711x(80)90128-7. [DOI] [PubMed] [Google Scholar]
- 44.McLaren DJ, James SL. Ultrastructural studies of the killing of schistosomula of Schistosoma mansoni by activated macrophages in vitro. Parasite Immunol. 1985;7:315–331. doi: 10.1111/j.1365-3024.1985.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 45.Huang SC, et al. Fatty acid oxidation is essential for egg production by the parasitic flatworm Schistosoma mansoni. PLoS Pathog. 2012;8:e1002996. doi: 10.1371/journal.ppat.1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennet E-M, Behm CA, Bryant C. Comparative Biochemistry of Parasitic Helminths. Chapman and Hall; London: 1989. [Google Scholar]
- 47.Fry M, Jenkins DC. Nippostrongylus brasiliensis: The effect of mitochondrial inhibitors on life-cycle stages. Parasitology. 1984;88:163–177. doi: 10.1017/s0031182000054433. [DOI] [PubMed] [Google Scholar]
- 48.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504:46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 49.Xu W, Charles IG, Moncada S. Nitric oxide: Orchestrating hypoxia regulation through mitochondrial respiration and the endoplasmic reticulum stress response. Cell Res. 2005;15:63–65. doi: 10.1038/sj.cr.7290267. [DOI] [PubMed] [Google Scholar]
- 50.Sarti P, Forte E, Mastronicola D, Giuffrè A, Arese M. Cytochrome c oxidase and nitric oxide in action: Molecular mechanisms and pathophysiological implications. Biochim Biophys Acta. 2012;1817:610–619. doi: 10.1016/j.bbabio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Sen S, Kawahara B, Chaudhuri G. Mitochondrial-associated nitric oxide synthase activity inhibits cytochrome c oxidase: Implications for breast cancer. Free Radic Biol Med. 2013;57:210–220. doi: 10.1016/j.freeradbiomed.2012.10.545. [DOI] [PubMed] [Google Scholar]
- 52.Aguirre E, Rodríguez-Juárez F, Bellelli A, Gnaiger E, Cadenas S. Kinetic model of the inhibition of respiration by endogenous nitric oxide in intact cells. Biochim Biophys Acta. 2010;1797:557–565. doi: 10.1016/j.bbabio.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 53.Zhong ZR, Xu YX, Gong JY, Luo QL. Isolation and identification of viability of Schistosoma japonicum eggs with high purity. Chin J Zoonoses. 2012;28:209–212. [Google Scholar]
- 54.Sarvel AK, Kusel JR, Araújo N, Coelho P, Katz N. Comparison between morphological and staining characteristics of live and dead eggs of Schistosoma mansoni. Mem Inst Oswaldo Cruz. 2006;101:289–292. doi: 10.1590/s0074-02762006000900045. [DOI] [PubMed] [Google Scholar]
- 55.Wynn TA, Eltoum I, Oswald IP, Cheever AW, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amiri P, et al. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 57.Bartley PB, et al. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Burke ML, et al. Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PLoS Negl Trop Dis. 2010;4:e598. doi: 10.1371/journal.pntd.0000598. [DOI] [PMC free article] [PubMed] [Google Scholar]