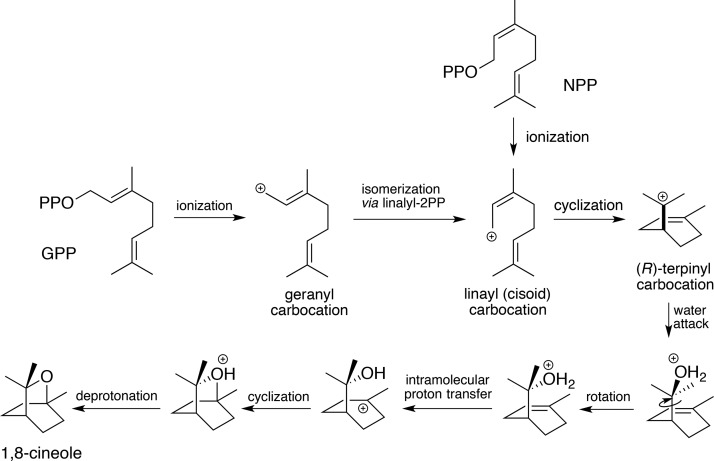
Figure 5.
Mechanistic proposal for bCinS. A schematic outline of a putative mechanism for the conversion of GPP and NPP to the 1,8 cineole product by bCinS. Taking into account the observed position and orientation of the bCinS ligands and adjacent water molecules, we propose that the (R)-terpinyl carbocation intermediate is formed, followed by the anti-addition of water, requiring a rotation step prior to hetercyclization.