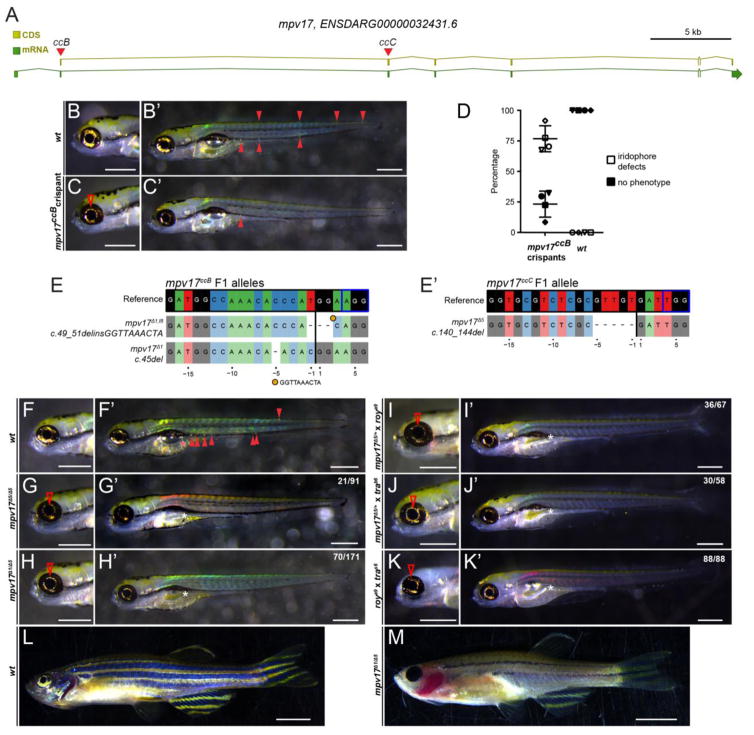
Figure 4.
A) Schematic of the zebrafish mpv17 locus, with coding sequence (CDS) and total mRNA-coding region including introns, red triangles depict sgRNA locations ccB and ccC in coding exons. (B,B′) Brightfield imaging of 5 dpf zebrafish embryos to visualize iridophores, anterior to the left; scale bars represent 500 μm. Wildtype (wt) control showing prominent iridophores surrounding the eye lens and as individual cells along the body (solid arrowheads). (C,C′) Representative Cas9 RNP-injected, somatic-mutant embryo (crispant) where the ORF of mpv17 was targeted with sgRNA ccB in coding exon 1; note diminished iridophores in eye (red arrowhead) and severe reduction of body iridophores. (D) Phenotype distribution in mpv17ccB crispants versus wt controls. (E,E′) Mutant mp 17 alleles isolated in the F1 generation injected with sgRNA ccB (left) and ccC (right) and recovered in complementation analysis; genomic reference sequence depicted on top, black vertical bar indicates Cas9 cleavage site, blue box depicts PAM. (F, F′) Wildtype (wt) forms iridophores in eye and along the body (red arrowhead) and properly inflates the swimbladder (red asterisks). (G–H) Homozygous mp 17Δ5 and trans-heterozygous mp 17Δ1/Δ5 embryos show severe reduction of iridophores. (I,J) The same phenotype occurs in embryos trans-heterozygous for mp 17Δ5 and roya9 (I, I′) or mp 17Δ5 and trab6 (J,J′) as confirmed by genotyping for mp 17Δ5 (n=4). (K,K′) Trans-heterozygous embryos for roya9 and trab6 also show severe iridophore reduction, establishing non-complementation between roya9 and trab6 as mutant alleles of mpv17. (L) 3 month old wildtype with iridophores in the eye and along the body and melanocytes form normally into longitudinal stripes. (M) Trans-heterozygous mp 17Δ1/Δ5 fish completely lack eye iridophores and most iridophores along the body axis. Melanocytes in the stripes are heavily reduced. Scale bars represent 5 mm. White asterisks in whole embryo views mark failed inflation of the swimbladder in all observed mpv17 mutant combinations.