Abstract
Lung squamous cell carcinoma (LUSC) accounts for a significant proportion of lung cancer and there have been few therapeutic alternatives for recurrent LUSC due to the lack of specific driver molecules. To investigate the prospective role of lncRNAs in the tumorigenesis and progression of LUSC, the aberrantly expressed lncRNAs were calculated based on The Cancer Genome Atlas RNA-seq data. Of 7589 lncRNAs with 504 LUSC cases, 884 lncRNAs were identified as being aberrantly expressed (|log2 fold change| >2 and adjusted P<0.05) by DESeq R. The top 10 lncRNAs with the highest diagnostic value were SFTA1P,LINC00968, LINC00961, LINC01572,RP1-78O14.1, FENDRR, LINC01314,LINC01272, GATA6-AS1, and MIR3945HG. In addition to the significant roles in the carcinogenesis of LUSC, several lncRNAs also played vital parts in the survival and progression of LUSC. SFTA1P, LINC01272, GATA6-AS1 and MIR3945HG were closely related to the survival time of LUSC. Furthermore, LINC01572 and LINC01314 could distinguish the LUSC at early stage from that at advanced stage. The prospective molecular assessment of key lncRNAs showed that a certain series of genes could be involved in the regulation network. Furthermore, the OncoPrint from cBioPortal indicated that 14% (69/501) LUSC cases with genetic alterations could be obtained, including amplification, deep deletion and mRNA upregulation. More interestingly, the cases with genetic alterations had a poorer survival as compared to those without alterations. Overall, the study propounds a potentiality for interpreting the pathogenesis and development of LUSC with lncRNAs, and provides a novel platform for searching for more capable diagnostic biomarkers for LUSC.
Keywords: lncRNAs, LUSC, biomarker, TCGA, tumorigenesis
INTRODUCTION
Lung cancer is the one of the leading causes of cancer deaths in the world. Among all lung cancers, more than 85% are categorized as non-small cell lung cancer (NSCLC), of which lung squamous cell carcinoma (LUSC) accounts for an approximate proportion of 30% [1–6]. Different from lung adenocarcinoma (LUAD), LUSC starts in squamous cells, which are slim, flat cells from histology, which look like fish scales. More importantly, the genetic and epigenetic profiles in the process of tumorigenesis and development vary strikingly between LUAD and LUSC [7–10]. There is a wide range of pivotal molecules verified for LUAD, which leads to great therapeutic improvement for recurrent or unresectable LUAD. Instead, there have been few therapeutic alternatives for recurrent LUSC due to the lack of specific driver molecules or mutations [11–15]. Hence, accurate indicators in the tumorigenesis and development of LUSC are urgently required.
To date, a number of prospective markers for LUSC have been identified; however, the pathogenesis of LUSC is sophisticated. Furthermore, sensitive and specific markers are lacking to identify LUSC in the early stage. Long non-coding RNAs (lncRNAs) have arisen as new master regulators of initiation, progression, and response to specific therapies in a broad variety of solid and hematological neoplasms [16–18]. LncRNAs have also been demonstrated to gain various functions in tumorigenesis of lung cancer. However, most of the studies concerned the general NSCLC, but few focused on LUSC [19]. Thus, identification of LUSC-related lncRNAs, and investigation of their clinical roles and molecular mechanisms are essential for understanding the development and progression of LUSC.
The Cancer Genome Atlas (TCGA) database of LUSC has facilitated the analysis on the high throughput data of various genomic alterations, including non-coding RNAs. The aberrantly expressed genes were identified for LUSC based on TCGA data and those genes that highly mutated were highlighted [20]. The clinical role of the most significantly altered microRNAs was also studied in TCGA LUSC cohort [21]. Most recently, the lncRNA alteration frequencies, but not the expression levels, were investigated by cBioPortal with 504 cases of LUSC, as well as LUAD from TCGA database [22]. Another study also compared the lncRNA profiling in LUAD and LUSC with data from TCGA and Gene Expression Omnibus (GEO). However, the concern of this study was the distinct lncRNA expression pattern between LUAD and LUSC. Furthermore, only the paired tissue samples of RNA-sequencing (RNA-Seq) from TCGA (16 pairs) were analyzed. Even the authors validated their findings with microarray data from GEO (GSE19188), only a small number of cases were involved [23]. Thus, in the current study, we calculated the 884 aberrantly expressed lncRNAs from 7589 lncRNAs in 502 LUSC cases. We further selected the top 10 lncRNAs to evaluate their clinicopathological value and potential mechanism for LUSC.
RESULTS
Aberrantly expressed lncRNAs based on TCGA data in LUSC
The expression level of each lncRNA transformed with log2 was calculated by DESeq R. Following the calculating criteria, we achieved 884 aberrantly expressed lncRNAs (Figure 1) in LUSC, including 669 highly and 215 lowly expressed lncRNAs. All the aberrantly expressed lncRNAs were sent for ROC analysis and we listed the top 75 lncRNAs obtaining over 0.95 for the area under ROC curve (AUC) (Table 1), which demonstrated that these lncRNAs might play essential roles in the occurrence of LUSC and had high diagnostic value for LUSC patients.
Figure 1. Volcano plot of the aberrantly expressed lncRNAs between LUSC and para-tumorous lung tissues.
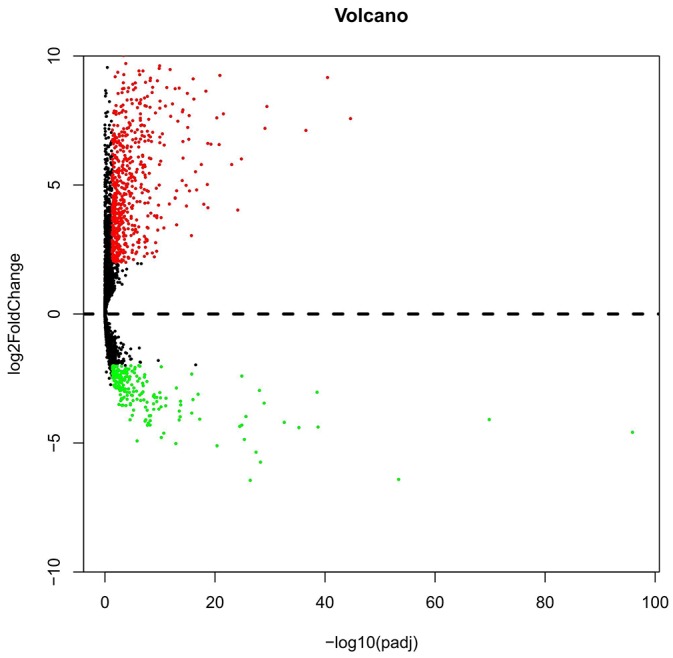
Red dots indicate high expression and green dots indicate low expression of lncRNAs. Black dots show the lncRNAs with expression of |log2FC|<2. The X axis represents an adjusted FDR and the Y axis represents the value of log2FC. Aberrantly expressed lncRNAs were calculated by DESeq R. Altogether, 669 high and 215 low expressed lncRNAs were achieved. This volcano plot was conducted by the ggplot2 package of R language.
Table 1. Analysis results of 75 lncRNAs gaining the most significant diagnostic value for LUSC (AUC >0.95).
| LncRNA | AUC | FC | Log2FC | P-value | Adjusted P-value |
|---|---|---|---|---|---|
| SFTA1P | 0.998415 | 0.041652365 | -4.585457785 | 3.1E-100 | 1.3E-96 |
| LINC00968 | 0.997398 | 0.04726163 | -4.403186805 | 1.18E-38 | 5.55E-36 |
| LINC00961 | 0.996585 | 0.100222391 | -3.318723236 | 1.07E-18 | 1.01E-16 |
| LINC01572 | 0.996341 | 9.626953056 | 3.267079255 | 6.51E-07 | 9.65E-06 |
| RP1-78O14.1 | 0.995122 | 0.054413873 | -4.199881671 | 6E-36 | 2.53E-33 |
| FENDRR | 0.994105 | 0.05863219 | -4.09216325 | 6.64E-74 | 1.4E-70 |
| LINC01314 | 0.993983 | 0.047958874 | -4.382058392 | 2.62E-42 | 1.85E-39 |
| LINC01272 | 0.992194 | 0.122249416 | -3.032100523 | 4.58E-42 | 2.76E-39 |
| GATA6-AS1 | 0.991788 | 0.105033789 | -3.251074585 | 1.82E-12 | 8.09E-11 |
| MIR3945HG | 0.991463 | 0.050535778 | -4.306551063 | 6.68E-28 | 1.34E-25 |
| LINC00607 | 0.990975 | 0.121229481 | -3.044187518 | 2.48E-12 | 1.05E-10 |
| PCAT19 | 0.990772 | 0.128341572 | -2.961939541 | 2.91E-31 | 8.19E-29 |
| AC018647.3 | 0.99069 | 0.077664175 | -3.686606922 | 3.88E-12 | 1.56E-10 |
| RP11-108L7.15 | 0.990284 | 8.687313032 | 3.118910023 | 3.92E-05 | 0.00038 |
| AC006273.4 | 0.98817 | 0.125905728 | -2.989584173 | 1.1E-07 | 1.86E-06 |
| LINC00702 | 0.987357 | 0.115641102 | -3.112273834 | 1.09E-19 | 1.18E-17 |
| AC109642.1 | 0.987275 | 0.091201867 | -3.454792838 | 3.57E-32 | 1.16E-29 |
| LINC01197 | 0.986056 | 0.155580728 | -2.684264729 | 6.63E-09 | 1.42E-07 |
| CTB-193M12.5 | 0.985405 | 5.156248886 | 2.366321903 | 7.43E-11 | 2.45E-09 |
| LINC00511 | 0.985121 | 16.33122057 | 4.029560714 | 4.14E-27 | 7.29E-25 |
| RP11-672A2.4 | 0.984796 | 0.1040007 | -3.265334859 | 1.55E-13 | 8.08E-12 |
| RP11-434D9.1 | 0.982803 | 0.073633018 | -3.763503358 | 4.61E-16 | 3.04E-14 |
| LINC00261 | 0.98256 | 0.095008585 | -3.395798301 | 1.02E-11 | 3.81E-10 |
| C14orf132 | 0.980811 | 0.188757476 | -2.405394311 | 6.34E-28 | 1.34E-25 |
| FAM83H-AS1 | 0.980649 | 8.212432406 | 3.037809591 | 2.14E-18 | 1.89E-16 |
| Z83851.4 | 0.979063 | 6.000254472 | 2.585023687 | 3.61E-08 | 6.63E-07 |
| RP11-532F6.3 | 0.977275 | 0.195894607 | -2.351850411 | 2.53E-09 | 5.97E-08 |
| SLC2A1-AS1 | 0.976583 | 10.24741912 | 3.357188699 | 7.82E-10 | 2.12E-08 |
| RP11-161I6.2 | 0.976319 | 65.93239774 | 6.042915643 | 8.46E-17 | 6.38E-15 |
| LINC01290 | 0.975079 | 0.187150523 | -2.417729014 | 7.79E-06 | 9.09E-05 |
| RP11-796E10.1 | 0.974876 | 54.56086965 | 5.769794735 | 5.57E-09 | 1.23E-07 |
| RP11-513N24.1 | 0.974429 | 0.174994013 | -2.514622534 | 3E-06 | 3.8E-05 |
| RP11-401P9.4 | 0.974144 | 0.176841689 | -2.499469676 | 2.8E-08 | 5.26E-07 |
| AC068831.16 | 0.974002 | 35.54345896 | 5.151512181 | 8.37E-07 | 1.19E-05 |
| AC007405.4 | 0.973778 | 0.145766461 | -2.778269281 | 4.91E-09 | 1.09E-07 |
| LINC00472 | 0.973494 | 0.215066069 | -2.217148164 | 7.91E-07 | 1.14E-05 |
| OGFRP1 | 0.973453 | 6.108043922 | 2.610710436 | 6.7E-06 | 7.99E-05 |
| RP5-1159O4.2 | 0.973006 | 0.207307461 | -2.270156058 | 2.37E-05 | 0.000245 |
| RP11-560J1.2 | 0.972823 | 6.169801561 | 2.625224089 | 0.000237 | 0.001837 |
| CTD-2527I21.15 | 0.972193 | 97.72416271 | 6.610643414 | 1.59E-21 | 2.03E-19 |
| RP11-540A21.2 | 0.972112 | 6.673744623 | 2.738496482 | 1.68E-05 | 0.000178 |
| CASC9 | 0.971827 | 190.5530192 | 7.574048657 | 2.21E-48 | 2.33E-45 |
| RP11-12G12.7 | 0.971461 | 5.065939834 | 2.340829943 | 8.1E-10 | 2.15E-08 |
| RP11-613D13.8 | 0.971014 | 0.069766914 | -3.841313162 | 1.81E-18 | 1.66E-16 |
| RP11-245D16.4 | 0.970729 | 6.489351573 | 2.698074329 | 2.55E-05 | 0.000261 |
| RP11-473M20.9 | 0.970567 | 0.227433967 | -2.13648036 | 2.47E-07 | 3.9E-06 |
| RP4-758J18.13 | 0.970323 | 4.008078031 | 2.002910596 | 1.49E-05 | 0.000162 |
| LINC00519 | 0.970262 | 64.45033831 | 6.010116026 | 8.24E-28 | 1.58E-25 |
| RP11-435O5.2 | 0.968209 | 4.229037176 | 2.080329243 | 4.79E-05 | 0.00045 |
| RP11-396C23.2 | 0.967436 | 8.242877049 | 3.043147976 | 1.3E-06 | 1.77E-05 |
| RP11-284N8.3 | 0.966257 | 0.199304014 | -2.326957327 | 1.85E-18 | 1.66E-16 |
| RP11-236L14.2 | 0.966095 | 0.205476098 | -2.28295751 | 4.32E-05 | 0.000413 |
| PVT1 | 0.965851 | 5.393469549 | 2.431213639 | 1.1E-11 | 4.05E-10 |
| AC005537.2 | 0.96457 | 36.0538193 | 5.172080192 | 1.28E-16 | 9.04E-15 |
| AC006273.5 | 0.960363 | 0.163091113 | -2.616249925 | 9.01E-10 | 2.33E-08 |
| CTD-2626G11.2 | 0.959915 | 0.137024821 | -2.86749085 | 1.73E-15 | 1.03E-13 |
| CTD-2245E15.3 | 0.95955 | 0.188508673 | -2.40729719 | 9.76E-08 | 1.67E-06 |
| RP11-344B5.2 | 0.959062 | 0.248593902 | -2.008137185 | 1.43E-06 | 1.93E-05 |
| RP11-624L4.1 | 0.958899 | 13.3036348 | 3.733748566 | 1.33E-12 | 6.18E-11 |
| CTA-989H11.1 | 0.956907 | 5.557899999 | 2.474539877 | 4.67E-05 | 0.000439 |
| RP11-353N14.2 | 0.956785 | 15.46687569 | 3.951109896 | 6.61E-06 | 7.91E-05 |
| CARMN | 0.955728 | 0.249734448 | -2.001533255 | 3.89E-08 | 7.05E-07 |
| AC006129.1 | 0.955403 | 0.174046529 | -2.522455054 | 3.18E-06 | 3.99E-05 |
| RP11-776H12.1 | 0.955322 | 55.44550471 | 5.792998592 | 2.66E-20 | 3.03E-18 |
| RP11-244M2.1 | 0.955078 | 27.41409845 | 4.776846124 | 1.56E-15 | 9.48E-14 |
| RP13-463N16.6 | 0.954468 | 93.40291145 | 6.545395616 | 3.44E-13 | 1.71E-11 |
| RP11-546J1.1 | 0.953899 | 5.824459136 | 2.542124086 | 0.003303 | 0.01729 |
| MIR100HG | 0.953777 | 0.20365128 | -2.295827214 | 1.89E-05 | 0.0002 |
| RP11-1038A11.3 | 0.95337 | 27.31391595 | 4.77156426 | 4.97E-18 | 4.28E-16 |
| RP11-429J17.7 | 0.952801 | 5.763401812 | 2.526920605 | 0.000358 | 0.002668 |
| RP11-357P18.2 | 0.951947 | 0.123202366 | -3.020898138 | 5.65E-09 | 1.24E-07 |
| RP5-899E9.1 | 0.951825 | 0.246349573 | -2.021221126 | 0.000107 | 0.000915 |
| RP4-616B8.5 | 0.950524 | 6.546508194 | 2.710725601 | 0.000894 | 0.005807 |
| LINC00924 | 0.950159 | 0.185515247 | -2.430390334 | 2.2E-06 | 2.86E-05 |
| RP11-7F17.3 | 0.950037 | 0.203290027 | -2.298388653 | 8.14E-06 | 9.4E-05 |
FC: fold change
Clinical value of the top 10 aberrantly expressed lncRNAs in LUSC
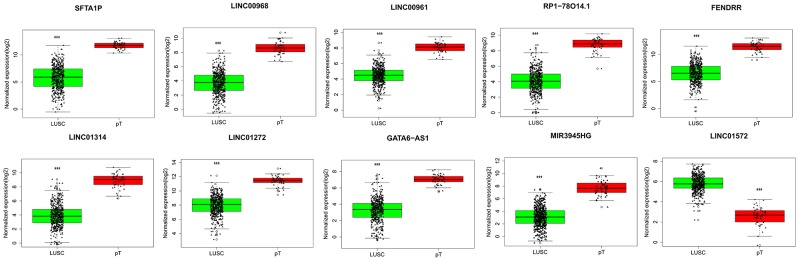
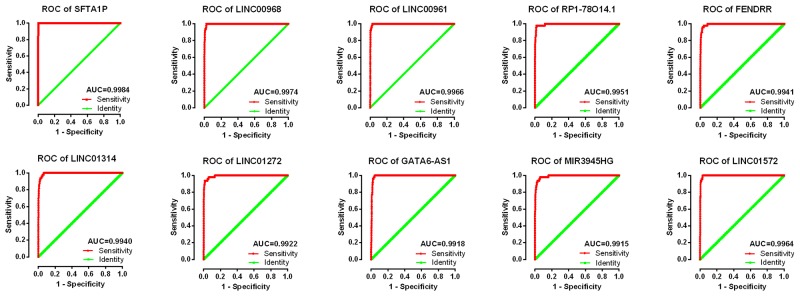
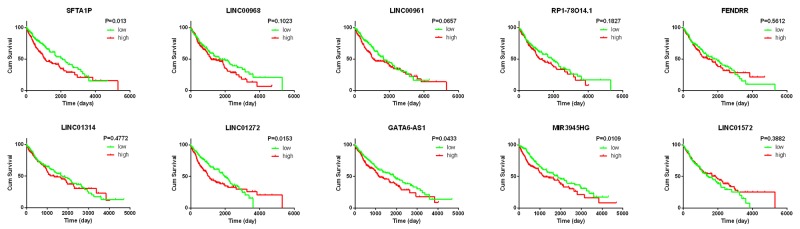
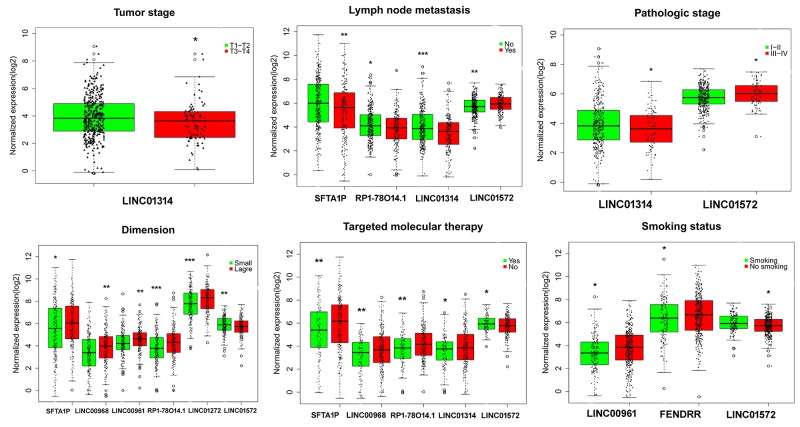
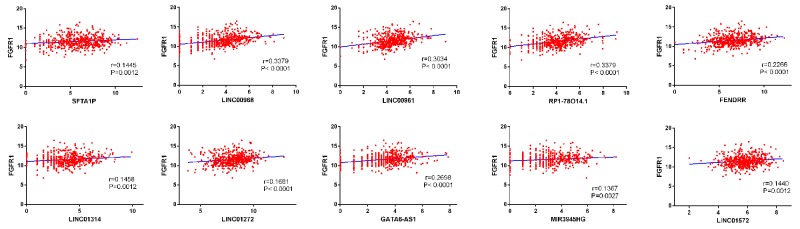
The top 10 aberrantly expressed lncRNAs (Table 2) were selected for further analysis, including Surfactant associated 1 (SFTA1P), LINC00968, LINC00961, LINC01572, RP1-78O14.1, FOXF1 adjacent non-coding developmental regulatory RNA (FENDRR), LINC01314, LINC01272, GATA6-AS1, and MIR3945HG. The level of LINC01572 was remarkably higher in the LUSC than that in the para-tumorous lung tissues. On the contrary, the other nine lncRNAs were all obviously downregulated in LUSC tissues (Figure 2). All these 10 aberrantly expressed lncRNAs showed high diagnostic values to distinguish LUSC from non-cancerous lung tissues with AUC all more than 0.99 (Figure 3). Survival analyses showed that SFTA1P, LINC01272, GATA6-AS1 and MIR3945HG were significantly related to the survival time of LUSC (Figure 4). Further, the multivariate cox analysis showed that SFTA1P might be an independent prognostic indicator for LUSC (P=0.019, Supplementary Table 1). When concerning the relationship between these 10 lncRNAs and the progression of LUSC, several lncRNAs were closely related to some clinical parameters of LUSC (Table 3, Figure 5). Especially, the level of LINC01572 and LINC01314 could distinguish the LUSC patients in early-stage from the advanced-stage. Original data of FGFR1 was extracted from TCGA platform. Significantly positive correlations were noted between FGFR1 and ten-lncRNA (Figure 6).
Table 2. Characteristics of top 10 LncRNAs by the AUC size ranking.
| LncRNA | Ensemble | Location | Regulation | FC | AUC | CI | P-value |
|---|---|---|---|---|---|---|---|
| SFTA1P | ENSG00000225383 | 10p14 | Down | 0.041652365 | 0.9984 | 0.996, 1.000 | <0.001 |
| LINC00968 | ENSG00000246430 | 8q12.1 | Down | 0.04726163 | 0.9974 | 0.995, 1.000 | <0.001 |
| LINC00961 | ENSG00000235387 | 9p13.3 | Down | 0.100222391 | 0.9966 | 0.993, 1.000 | <0.001 |
| LINC01572 | ENSG00000261008 | 16q22.2 | Up | 9.626953056 | 0.9963 | 0.992, 1.000 | <0.001 |
| RP1-78O14.1 | ENSG00000257894 | 12q21.2 | Down | 0.054413873 | 0.9951 | 0.990, 1.000 | <0.001 |
| FENDRR | ENSG00000268388 | 16q24.1 | Down | 0.05863219 | 0.9941 | 0.989, 0.999 | <0.001 |
| LINC01314 | ENSG00000259417 | 15q25.1 | Down | 0.047958874 | 0.9940 | 0.989, 0.999 | <0.001 |
| LINC01272 | ENSG00000224397 | 20q13.13 | Down | 0.122249416 | 0.9922 | 0.985, 0.999 | <0.001 |
| GATA6-AS1 | ENSG00000266010 | 18q11.2 | Down | 0.105033789 | 0.9918 | 0.985, 0.998 | <0.001 |
| MIR3945HG | ENSG00000251230 | 4q35.1 | Down | 0.050535778 | 0.9915 | 0.983, 0.999 | <0.001 |
FC: fold change; AUC: area under the curve
CI: confidence interval
Figure 2. Different expression of the top 10 lncRNAs between LUSC and para-tumorous lung tissues.
Red column indicates LUSC tissues, and green column indicates lung para-tumorous tissue (pT). The X axis indicates tissue types. The Y axis represents normalized expression of lncRNAs. This figure was drawn by ggplot2 package of R language. *: P<0.05, **: P<0.01, ***: P<0.001.
Figure 3. ROC curves of the top 10 lncRNAs sorted by AUC in LUSC.
Red represents sensitive curve, green indicates identify line. The X axis shows false positive rate, presented as “1-Specificity”. The Y axis indicates true positive rate, shown as “Sensitivity”. These curves were provided by GraphPad Prism 6.
Figure 4. K-M curves of the top 10 lncRNAs in LUSC.
Red line represents high level of a lncRNA, and green line represents low level. The X axis indicates overall survival time (day), and the Y axis indicates the survival rate. These curves were conducted by GraphPad Prism 6.
Table 3. Relationship between the expression of the top 10 lncRNAs and clinicopathological factors in LUSC from TCGA.
| LncRNA\factor | Dimension (small/large) | Smoking (no/yes) | T (T1/2 vs. T3/4) | N (no/yes) | M (no/yes) | Pathological stage (I/II vs III/IV) | Targeted molecular therapy (no/yes) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t | P | t | P | t | P | t | P | t | P | t | P | t | P | |
| SFTA1P | -2.236 | 0.026 | -1.097 | 0.273 | 1.681 | 0.093 | -2.670 | 0.008 | 1.182 | 0.238 | 0.020 | 0.984 | -2.542 | 0.011 |
| LINC00968 | -2.752 | 0.006 | -2.549 | 0.011 | 1.138 | 0.256 | -0.269 | 0.788 | 0.950 | 0.343 | 0.989 | 0.323 | -2.910 | 0.044 |
| LINC00961 | -3.169 | 0.002 | -1.806 | 0.072 | 1.903 | 0.058 | 1.635 | 0.103 | 0.416 | 0.678 | -0.553 | 0.581 | -0.209 | 0.835 |
| LINC01572 | 2.408 | 0.016 | 2.433 | 0.015 | -0.096 | 0.924 | 3.012 | 0.003 | 1.959 | 0.051 | -2.717 | 0.007 | 2.123 | 0.034 |
| RP1-78O14.1 | -3.597 | <0.001 | 1.020 | 0.308 | 0.087 | 0.930 | -2.250 | 0.025 | 0.644 | 0.520 | 1.137 | 0.246 | -2.634 | 0.009 |
| FENDRR | -1.058 | 0.290 | -1.991 | 0.047 | 1.812 | 0.071 | -0.588 | 0.536 | 0.603 | 0.547 | 1.133 | 0.258 | -1.497 | 0.135 |
| LINC01314 | -1.036 | 0.301 | -0.201 | 0.841 | 2.066 | 0.039 | -3.880 | <0.001 | 0.493 | 0.623 | 1.991 | 0.047 | -2.335 | 0.020 |
| LINC01272 | -3.333 | 0.001 | 0.070 | 0.994 | -0.672 | 0.502 | -1.189 | 0.235 | 1.430 | 0.153 | 0.131 | 0.896 | -1.367 | 0.172 |
| GATA6-AS1 | 0.424 | 0.672 | 0.996 | 0.320 | 0.343 | 0.732 | -0.623 | 0.534 | -0.336 | 0.737 | 0.761 | 0.447 | -1.716 | 0.087 |
| MIR3945HG | -1.730 | 0.084 | 1.161 | 0.246 | -0.118 | 0.907 | -1.580 | 0.115 | -0.517 | 0.605 | 1.371 | 0.171 | -1.869 | 0.062 |
T: tumor stage; N: lymph node; M: metastasis
Figure 5. Association between the expression of key lncRNAs and clinicopathological features in LUSC.
Statistical significance differences of several key lncRNAs were noted in various clinicopathological features: tumor stage (T1/T2 vs. T3/T4), lymph node metastasis (no vs. yes), pathological stage (I/II vs. III/IV), smoking status (no smoking vs. current smoking), targeted molecular therapy (no vs. yes). The X axis indicates different lncRNAs, and the Y axis indicates the normalized expression (log2). The plots were conducted by ggplot2 package of R language. *: P<0.05, **: P<0.01, ***: P<0.001.
Figure 6. Correlation between FGFR1 expression and lncRNAs in LUSC.
The expression of these lncRNAs were positively correlated with FGFR1 expression based on TCGA dataset.
Potential molecular mechanism of the top 10 aberrantly expressed lncRNAs in LUSC
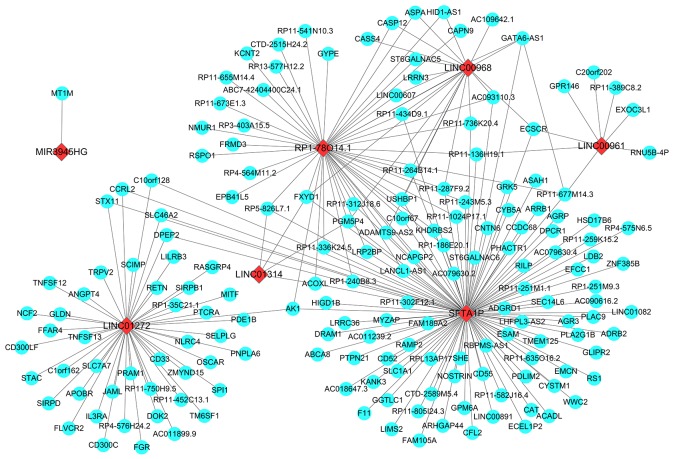
The co-expressed genes of all these ten key lncRNAs were determined by the WGCNA. As a result, 120 genes were revealed to be co-expressed with SFTA1P, and 47 genes were discovered to have co-expressed relationship with LINC01272, as well as the other key lncRNAs (46 genes for RP1-78O14.1, 18 for LINC00968, 8 for LINC00961, 4 for LINC01314, and 2 for GATA6-AS1 and 1 for MIR3945HG). Whereas the WGCNA showed no gene being co-expressed with FENDRR or LINC01572 (Figure 7).
Figure 7. Prospective gene networks of the 10 top differentially expressed lncRNAs.
To explore the regulation network of the key lncRNAs, the co-expressed genes of those key down-regulated lncRNAs were screened out by WGCNA. Red diamonds showed the key lncRNAs and blue balls are for key lncRNAs co-expressed mRNAs.
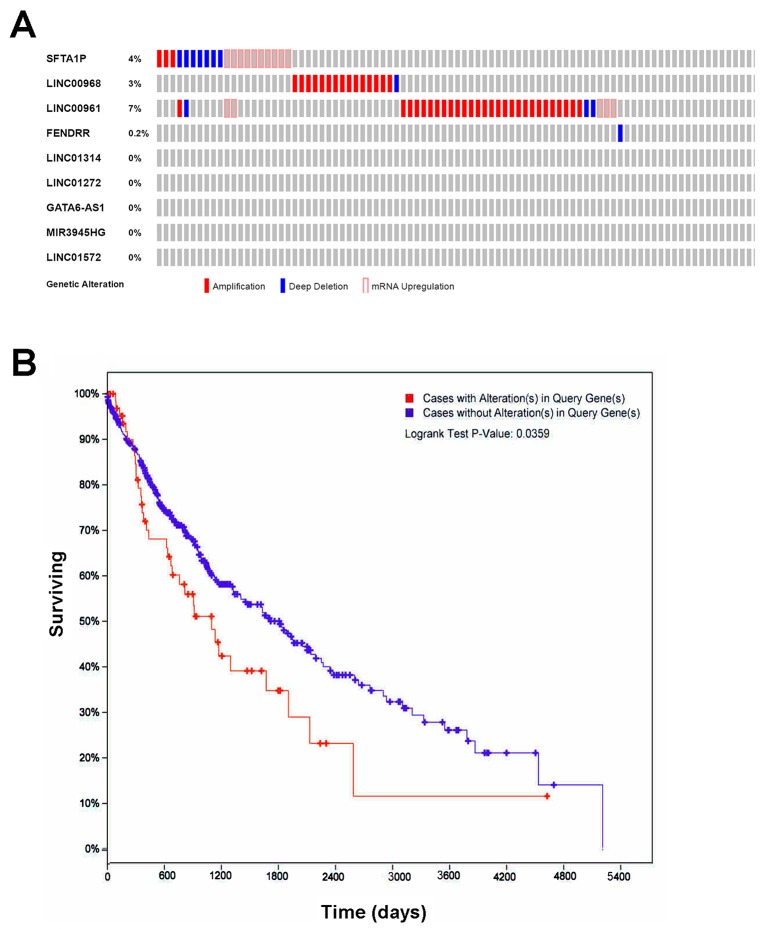
The OncoPrint from cBioPortal showed that 14% (69/501) cases with genetic alterations could be obtained (Figure 8A), except RP1-78O14.1, whose data were not available in cBioPortal. And only SFTA1P, LINC00968, LINC00961, and FENDRR had genetic alterations, including amplification, deep deletion and mRNA upregulation. More interestingly, the cases with genetic alterations had a poorer survival as compared to those without alterations (P=0.0359, Figure 8B). CBioPortal also provided the probable co-occurrence of these top 10 lncRNAs. As Table 4 showed, there was a tendency towards co-occurrence between SFTA1P and LINC00961 in LUSC.
Figure 8. The genetic alterations and their prognostic value of the lncRNAs in LUSC.
(A) Genetic alterations. Red represents amplification, blue represents deep deletion and pink represents mRNA up-regulation. Genetic alterations were found in 69 of 501 LUSC patients (14%). The aberrant expression threshold was defined as z-score ± 2.0 from the TCGA RNA Seq V2 data. This OncoPrint was conducted by cBioPortal. (B) K-M curve between groups with alterations and without alterations. Red line represents cases with alterations, and blue line represents cases without. The X axis indicates overall survival time (days), and the Y axis indicates the survival rate. Kaplan-Meier test was performed. These curves were generated by cBioPortal.
Table 4. Results of mutual exclusivity and co-occurrence analysis by cBioPortal.
| Gene A | Gene B | P-value | Log odds ratio | Association |
|---|---|---|---|---|
| SFTA1P | LINC00968 | 0.515821057 | -Infinity | Tendency towards mutual exclusivity |
| SFTA1P | LINC00961 | 0.04745977 | 1.2549926238226372 | Tendency towards co-occurrence(Significant) |
| SFTA1P | LINC01572 | 1 | Infinity | Tendency towards co-occurrence |
| SFTA1P | FENDRR | 0.96007984 | -Infinity | Tendency towards mutual exclusivity |
| SFTA1P | LINC01314 | 1 | Infinity | Tendency towards co-occurrence |
| SFTA1P | LINC01272 | 1 | Infinity | Tendency towards co-occurrence |
| SFTA1P | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| SFTA1P | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| LINC00968 | LINC00961 | 0.297586666 | -Infinity | Tendency towards mutual exclusivity |
| LINC00968 | LINC01572 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00968 | FENDRR | 0.968063872 | -Infinity | Tendency towards mutual exclusivity |
| LINC00968 | LINC01314 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00968 | LINC01272 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00968 | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00968 | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| LINC00961 | LINC01572 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00961 | FENDRR | 0.928143713 | -Infinity | Tendency towards mutual exclusivity |
| LINC00961 | LINC01314 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00961 | LINC01272 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00961 | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| LINC00961 | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| LINC01572 | FENDRR | 1 | Infinity | Tendency towards co-occurrence |
| LINC01572 | LINC01314 | 1 | Infinity | Tendency towards co-occurrence |
| LINC01572 | LINC01272 | 1 | Infinity | Tendency towards co-occurrence |
| LINC01572 | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| LINC01572 | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| FENDRR | LINC01314 | 1 | Infinity | Tendency towards co-occurrence |
| FENDRR | LINC01272 | 1 | Infinity | Tendency towards co-occurrence |
| FENDRR | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| FENDRR | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| LINC01314 | LINC01272 | 1 | Infinity | Tendency towards co-occurrence |
| LINC01314 | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| LINC01314 | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| LINC01272 | GATA6-AS1 | 1 | Infinity | Tendency towards co-occurrence |
| LINC01272 | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
| GATA6-AS1 | MIR3945HG | 1 | Infinity | Tendency towards co-occurrence |
The query contains 5 gene pairs with mutually exclusive alterations (none significant), and 31 gene pairs with co-occurrent alterations (1 significant).
Log odds ratio > 0: Association towards co-occurrence
Log odds ratio <= 0: Association towards mutual exclusivity
P-value < 0.05: Significant association
P-value: Derived from Fisher Exact Test
Log odds ratio: Quantifies how strongly the presence or absence of alterations in gene A are associated with the presence or absence of alterations in gene B in the selected tumors
As a result, the STA1P co-expressed genes were most enriched in lysosome and LINC01272 co-expressed genes were most significantly involved in integral component of membrane. Meanwhile, the most enriched GO terms for mRNAs co-expressed with RP1-78O14.1 was actomyosin structure organization. The result was shown in Table 5. Additionally, we also analyzed the most enriched GO terms within all the mRNAs co-expressed with these lncRNAs. Consequently, plasma membrane was revealed to be the most GO terms and the result was showed in Table 6.
Table 5. Significant GO terms based the co-expressed genes with each lncRNA.
| Category | Term | Count | % | P-value | Fold enrichment | Bonferroni | Benjamini | FDR |
|---|---|---|---|---|---|---|---|---|
| SFTA1P | ||||||||
| GOTERM_CC_DIRECT | GO:0005764∼lysosome | 6 | 7.89 | 7.53E-04 | 8.20 | 0.06 | 0.06 | 0.81 |
| GOTERM_CC_DIRECT | GO:0005886∼plasma membrane | 24 | 31.58 | 0.002627 | 1.80 | 0.21 | 0.11 | 2.81 |
| GOTERM_CC_DIRECT | GO:0031225∼anchored component of membrane | 4 | 5.26 | 0.005591 | 10.93 | 0.39 | 0.15 | 5.90 |
| GOTERM_CC_DIRECT | GO:0016021∼integral component of membrane | 25 | 32.89 | 0.022222 | 1.50 | 0.86 | 0.39 | 21.65 |
| GOTERM_MF_DIRECT | GO:0009055∼electron carrier activity | 3 | 3.95 | 0.030268 | 10.82 | 0.99 | 0.99 | 30.54 |
| GOTERM_BP_DIRECT | GO:0016337∼single organismal cell-cell adhesion | 3 | 3.95 | 0.037736 | 9.59 | 1.00 | 1.00 | 40.73 |
| GOTERM_BP_DIRECT | GO:0045730∼respiratory burst | 2 | 2.63 | 0.038785 | 49.68 | 1.00 | 1.00 | 41.61 |
| GOTERM_CC_DIRECT | GO:0043197∼dendritic spine | 3 | 3.95 | 0.040391 | 9.27 | 0.97 | 0.52 | 36.08 |
| GOTERM_MF_DIRECT | GO:0052890∼oxidoreductase activity, acting on the CH-CH group of donors, with a flavin as acceptor | 2 | 2.63 | 0.044389 | 43.28 | 1.00 | 0.96 | 41.63 |
| GOTERM_BP_DIRECT | GO:0043149∼stress fiber assembly | 2 | 2.63 | 0.04462 | 43.06 | 1.00 | 0.99 | 46.25 |
| GOTERM_MF_DIRECT | GO:0003995∼acyl-CoA dehydrogenase activity | 2 | 2.63 | 0.047279 | 40.58 | 1.00 | 0.90 | 43.69 |
| GOTERM_BP_DIRECT | GO:0033539∼fatty acid beta-oxidation using acyl-CoA dehydrogenase | 2 | 2.63 | 0.053306 | 35.88 | 1.00 | 0.99 | 52.53 |
| GOTERM_BP_DIRECT | GO:0019370∼leukotriene biosynthetic process | 2 | 2.63 | 0.059055 | 32.29 | 1.00 | 0.98 | 56.30 |
| GOTERM_CC_DIRECT | GO:0031674∼I band | 2 | 2.63 | 0.070735 | 26.86 | 1.00 | 0.66 | 54.90 |
| GOTERM_BP_DIRECT | GO:0046686∼response to cadmium ion | 2 | 2.63 | 0.073276 | 25.83 | 1.00 | 0.99 | 64.47 |
| GOTERM_MF_DIRECT | GO:0004857∼enzyme inhibitor activity | 2 | 2.63 | 0.086845 | 21.64 | 1.00 | 0.96 | 65.95 |
| GOTERM_MF_DIRECT | GO:0000062∼fatty-acyl-CoA binding | 2 | 2.63 | 0.086845 | 21.64 | 1.00 | 0.96 | 65.95 |
| LINC01272 | ||||||||
| GOTERM_CC_DIRECT | GO:0016021∼integral component of membrane | 24 | 57.14 | 0.000105 | 2.07 | 0.01 | 0.01 | 0.10 |
| GOTERM_BP_DIRECT | GO:0050900∼leukocyte migration | 5 | 11.90 | 0.000131 | 18.60 | 0.03 | 0.03 | 0.17 |
| GOTERM_CC_DIRECT | GO:0005886∼plasma membrane | 21 | 50.00 | 0.000134 | 2.27 | 0.01 | 0.00 | 0.13 |
| GOTERM_BP_DIRECT | GO:0050776∼regulation of immune response | 5 | 11.90 | 0.000552 | 12.75 | 0.11 | 0.06 | 0.70 |
| GOTERM_CC_DIRECT | GO:0005887∼integral component of plasma membrane | 10 | 23.81 | 0.003022 | 3.14 | 0.16 | 0.05 | 2.94 |
| GOTERM_BP_DIRECT | GO:0007165∼signal transduction | 8 | 19.05 | 0.010545 | 3.13 | 0.90 | 0.54 | 12.62 |
| GOTERM_BP_DIRECT | GO:0007169∼transmembrane receptor protein tyrosine kinase signaling pathway | 3 | 7.14 | 0.017961 | 14.18 | 0.98 | 0.63 | 20.60 |
| GOTERM_MF_DIRECT | GO:0005164∼tumor necrosis factor receptor binding | 2 | 4.76 | 0.058461 | 32.34 | 1.00 | 1.00 | 48.36 |
| GOTERM_BP_DIRECT | GO:0002376∼immune system process | 2 | 4.76 | 0.060391 | 31.30 | 1.00 | 0.94 | 54.75 |
| GOTERM_BP_DIRECT | GO:0045087∼innate immune response | 4 | 9.52 | 0.063923 | 4.22 | 1.00 | 0.91 | 56.87 |
| GOTERM_BP_DIRECT | GO:0001525∼angiogenesis | 3 | 7.14 | 0.082417 | 6.11 | 1.00 | 0.93 | 66.54 |
| RP1-78O14.1 | ||||||||
| GOTERM_BP_DIRECT | GO:0031032∼actomyosin structure organization | 2 | 8.33 | 0.019131 | 95.68 | 0.79 | 0.79 | 18.65 |
| GOTERM_BP_DIRECT | GO:0006821∼chloride transport | 2 | 8.33 | 0.028223 | 64.58 | 0.90 | 0.69 | 26.35 |
| GOTERM_MF_DIRECT | GO:0008092∼cytoskeletal protein binding | 2 | 8.33 | 0.039095 | 46.89 | 0.84 | 0.84 | 31.40 |
| GOTERM_CC_DIRECT | GO:0019898∼extrinsic component of membrane | 2 | 8.33 | 0.065433 | 27.78 | 0.87 | 0.87 | 43.81 |
| GOTERM_MF_DIRECT | GO:0005200∼structural constituent of cytoskeleton | 2 | 8.33 | 0.087494 | 20.46 | 0.99 | 0.88 | 57.91 |
Table 6. Significant GO terms based the all the mRNAs co-expressed with lncRNAs.
| Category | Term | Count | % | P-Value | Fold enrichment | Bonferroni | Benjamini | FDR |
|---|---|---|---|---|---|---|---|---|
| GOTERM_CC_DIRECT | GO:0005886∼plasma membrane | 45 | 34.35115 | 1.82E-05 | 1.82569 | 0.002129 | 0.002129 | 0.020817 |
| GOTERM_CC_DIRECT | GO:0016021∼integral component of membrane | 52 | 39.69466 | 2.27E-05 | 1.683908 | 0.002657 | 0.001329 | 0.025982 |
| GOTERM_BP_DIRECT | GO:0050900∼leukocyte migration | 6 | 4.580153 | 0.000705 | 8.426899 | 0.309001 | 0.309001 | 1.017256 |
| GOTERM_BP_DIRECT | GO:0045730∼respiratory burst | 3 | 2.290076 | 0.002471 | 39.5416 | 0.726545 | 0.47707 | 3.523245 |
| GOTERM_CC_DIRECT | GO:0005887∼integral component of plasma membrane | 18 | 13.74046 | 0.003928 | 2.126832 | 0.369021 | 0.142294 | 4.397623 |
| GOTERM_BP_DIRECT | GO:0001525∼angiogenesis | 6 | 4.580153 | 0.00948 | 4.610232 | 0.993201 | 0.810559 | 12.89588 |
| GOTERM_BP_DIRECT | GO:0031032∼actomyosin structure organization | 3 | 2.290076 | 0.010552 | 19.03855 | 0.996147 | 0.750853 | 14.25337 |
| GOTERM_CC_DIRECT | GO:0005764∼lysosome | 6 | 4.580153 | 0.011147 | 4.438743 | 0.730576 | 0.279542 | 12.022 |
| GOTERM_MF_DIRECT | GO:0009055∼electron carrier activity | 4 | 3.053435 | 0.013857 | 7.897544 | 0.954853 | 0.954853 | 16.29635 |
| GOTERM_MF_DIRECT | GO:0005102∼receptor binding | 7 | 5.343511 | 0.013998 | 3.523692 | 0.956257 | 0.790851 | 16.44801 |
| GOTERM_BP_DIRECT | GO:0050776∼regulation of immune response | 5 | 3.816794 | 0.019727 | 4.813116 | 0.999971 | 0.876075 | 25.08463 |
| GOTERM_BP_DIRECT | GO:0008277∼regulation of G-protein coupled receptor protein signaling pathway | 3 | 2.290076 | 0.021302 | 13.18053 | 0.999987 | 0.847489 | 26.8108 |
| GOTERM_CC_DIRECT | GO:0072557∼IPAF inflammasome complex | 2 | 1.526718 | 0.029285 | 66.87706 | 0.969117 | 0.50118 | 28.79674 |
| GOTERM_CC_DIRECT | GO:0031225∼anchored component of membrane | 4 | 3.053435 | 0.029629 | 5.918324 | 0.970371 | 0.443729 | 29.08439 |
| GOTERM_MF_DIRECT | GO:0004046∼aminoacylase activity | 2 | 1.526718 | 0.032953 | 59.23158 | 0.999412 | 0.916227 | 34.7645 |
| GOTERM_MF_DIRECT | GO:0001665∼alpha-N-acetylgalactosaminide alpha-2,6-sialyltransferase activity | 2 | 1.526718 | 0.032953 | 59.23158 | 0.999412 | 0.916227 | 34.7645 |
| GOTERM_BP_DIRECT | GO:0007165∼signal transduction | 13 | 9.923664 | 0.035075 | 1.918613 | 1 | 0.930938 | 40.40209 |
| GOTERM_CC_DIRECT | GO:0031256∼leading edge membrane | 2 | 1.526718 | 0.046447 | 41.79817 | 0.996169 | 0.548389 | 41.9262 |
| GOTERM_BP_DIRECT | GO:0046470∼phosphatidylcholine metabolic process | 2 | 1.526718 | 0.056302 | 34.26939 | 1 | 0.977531 | 56.82828 |
| GOTERM_BP_DIRECT | GO:0032868∼response to insulin | 3 | 2.290076 | 0.057315 | 7.672251 | 1 | 0.967821 | 57.49532 |
| GOTERM_MF_DIRECT | GO:0016811∼hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds, in linear amides | 2 | 1.526718 | 0.059592 | 32.30813 | 0.999999 | 0.966959 | 54.30836 |
| GOTERM_BP_DIRECT | GO:0009312∼oligosaccharide biosynthetic process | 2 | 1.526718 | 0.061756 | 31.15399 | 1 | 0.964572 | 60.3076 |
| GOTERM_BP_DIRECT | GO:0045444∼fat cell differentiation | 3 | 2.290076 | 0.066642 | 7.041655 | 1 | 0.962572 | 63.20096 |
| GOTERM_BP_DIRECT | GO:0007171∼activation of transmembrane receptor protein tyrosine kinase activity | 2 | 1.526718 | 0.06718 | 28.55782 | 1 | 0.952007 | 63.50671 |
| GOTERM_CC_DIRECT | GO:0005856∼cytoskeleton | 6 | 4.580153 | 0.069766 | 2.703924 | 0.999789 | 0.652735 | 56.23642 |
| GOTERM_BP_DIRECT | GO:0007166∼cell surface receptor signaling pathway | 5 | 3.816794 | 0.07431 | 3.126769 | 1 | 0.955507 | 67.34839 |
| GOTERM_MF_DIRECT | GO:0052890∼oxidoreductase activity, acting on the CH-CH group of donors, with a flavin as acceptor | 2 | 1.526718 | 0.080379 | 23.69263 | 1 | 0.975777 | 65.63741 |
| GOTERM_BP_DIRECT | GO:0070374∼positive regulation of ERK1 and ERK2 cascade | 4 | 3.053435 | 0.080812 | 3.916501 | 1 | 0.957316 | 70.51973 |
| GOTERM_CC_DIRECT | GO:0005925∼focal adhesion | 6 | 4.580153 | 0.083008 | 2.565616 | 0.99996 | 0.675844 | 62.84951 |
| GOTERM_BP_DIRECT | GO:0043149∼stress fiber assembly | 2 | 1.526718 | 0.083264 | 22.84626 | 1 | 0.95202 | 71.63928 |
| GOTERM_MF_DIRECT | GO:0003995∼acyl-CoA dehydrogenase activity | 2 | 1.526718 | 0.085505 | 22.21184 | 1 | 0.96338 | 68.00047 |
| GOTERM_BP_DIRECT | GO:0033539∼fatty acid beta-oxidation using acyl-CoA dehydrogenase | 2 | 1.526718 | 0.099074 | 19.03855 | 1 | 0.967186 | 77.96049 |
| GOTERM_BP_DIRECT | GO:0001574∼ganglioside biosynthetic process | 2 | 1.526718 | 0.099074 | 19.03855 | 1 | 0.967186 | 77.96049 |
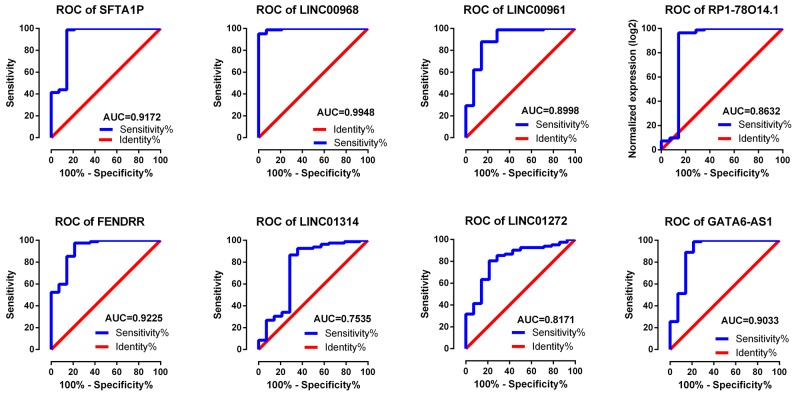
Validation of the expression and ROC of the eight lncRNAs with GEO data
One study was screened out from GEO datasets (GSE30219). The expression level of eight key lncRNAs, SFTA1P, LINC00968, LINC00961, RP1-78O14.1, FENDRR, LINC01314 and LINC01272, could be extracted from the dataset, among which the remarkably lower expression of SFTA1P, LINC00968, LINC00961, RP1-78O14.1, FENDRR, LINC01314 and LINC01272 could be observed, while predominantly higher expression of GATA6-AS1 was found in LUSC tissues (Table 7). The ROC curves of eight lncRNAs all indicated favorable diagnostic value of LUSC (Figure 9).
Table 7. Validation of expression and diagnostic value of eight lncRNAs in LUSC based on GEO dataset (GSE30219).
| Variable | pT | LUSC | T-test | ROC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | t | P | AUC | SE | 95% CI | P | |
| FENDRR | 14 | 5.214915 | 0.663845 | 82 | 4.295079 | 0.188372 | 7.254 | <0.0001 | 0.922 | 0.0437 | 0.850 - 0.967 | <0.0001 |
| GATA6-AS1 | 14 | 5.846385 | 0.939914 | 82 | 5.972000 | 13.29700 | 5.972 | <0.0001 | 0.903 | 0.0613 | 0.826 - 0.954 | <0.0001 |
| LINC00961 | 14 | 6.285801 | 0.370772 | 82 | 5.672997 | 0.255615 | 7.722 | <0.0001 | 0.900 | 0.0555 | 0.822 - 0.952 | <0.0001 |
| LINC00968 | 14 | 6.824595 | 1.210060 | 82 | 3.556648 | 0.449696 | 9.988 | <0.0001 | 0.995 | 0.0046 | 0.952 - 1.000 | <0.0001 |
| LINC01272 | 14 | 4.693669 | 0.253514 | 82 | 4.351741 | 0.286088 | 4.574 | <0.0001 | 0.817 | 0.0619 | 0.725 - 0.889 | <0.0001 |
| LINC01314 | 14 | 4.701564 | 0.272653 | 82 | 4.485906 | 0.155580 | 2.881 | 0.0120 | 0.753 | 0.0918 | 0.655 - 0.836 | 0.0058 |
| RP1-78O14.1 | 14 | 5.166360 | 1.060565 | 82 | 3.347113 | 0.398867 | 6.342 | <0.0001 | 0.863 | 0.0883 | 0.778 - 0.925 | <0.0001 |
| SFTA1P | 14 | 7.948137 | 1.428409 | 82 | 5.120226 | 0.715006 | 7.254 | <0.0001 | 0.917 | 0.0561 | 0.843 - 0.964 | <0.0001 |
pT: para-noncancerous tissue; LUSC: lung squamous cell carcinoma
Figure 9. Validation of ROC results of eight lncRNAs in LUSC based on GEO dataset.
Blue represents sensitive curve, red indicates identify line. The X axis shows false positive rate, presented as “100%- Specificity%”. The Y axis indicates true positive rate, shown as “Sensitivity”. These curves were performed by GraphPad Prism 6.
Validation based on clinical samples of LUSC
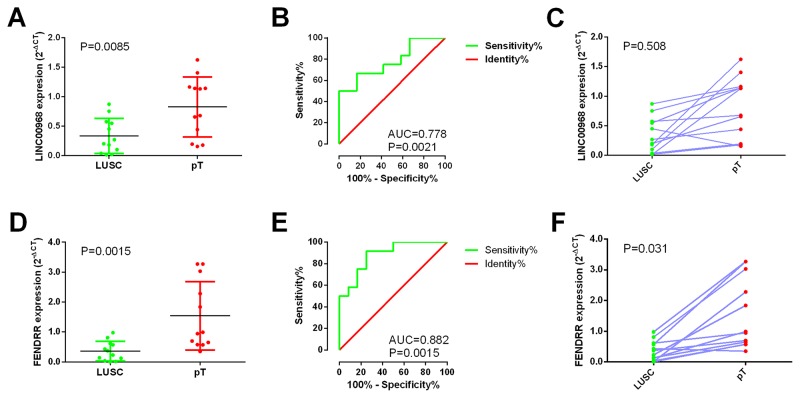
We performed real time RT-qPCR to confirm the expression of LINC00968 and FENDRR in the 12 paired clinical samples. In these patients, the mean expression level of LINC00968 was notably lower in LUSC tissues (0.3343±0.08582) than that of non-cancerous lung tissues (0.8258±0.1469; P=0.0085, Figure 10A). Moreover, the AUC of LINC00968 was 0.778 (P=0.0021, Figure 10B). However, there was no significant correlation between LINC00968 and the tumorigeneses of LUSC (P=0.508, Figure 10C). Meanwhile, the expression trend of FENDRR was similar to that of LINC00968 (P=0.0015, Figure 10D). The AUC of FENDRR is 0.882 (P=0.0015, Figure 10E). And we also assessed the relationship between FENDRR and the tumorigeneses of LUSC (P=0.031, Figure 10F).
Figure 10. Validation of LINC00968 and FENDRR based on 12 paired clinical samples of LUSC.
(A) The expression of LINC00968 between para-tumorous lung tissues (pT) and LUSC (RT-qPCR); (B) ROC curve of LINC00968; (C) The correlation of LINC00968 between para-tumorous lung tissues (pT) and LUSC; (D) The expression of FENDRR between para-tumorous lung tissues (pT) and LUSC (RT-qPCR); (E) ROC curve of FENDRR; (F) The correlation of FENDRR between para-tumorous lung tissues (pT) and LUSC. pT: para-noncancerous tissues.
Further analysis for the key lncRNAs expression in 22 types of cancers based on TCGA
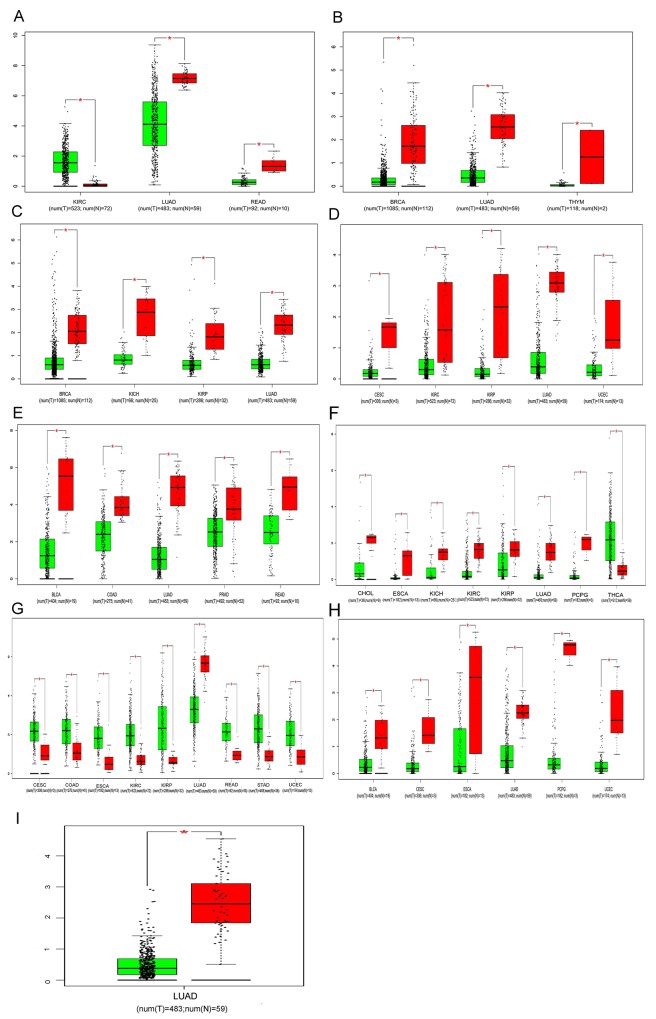
Based on the results derived from GEPIA, down-regulation of SFTA1P was found in the lung adenocarcinoma (LUAD) and rectal adenocarcinoma (READ), while the expression of SFTA1P was significantly up-regulated in clear cell kidney carcinoma (KIRC). As shown in the figures, the consistent results were found in breast cancer (BRCA), LUAD and thymoma (THYM), revealing that LINC00968 level was significant lower in these cancers compared with para-noncancerous tissues. consistent with the result in LUSC, the lower expression of LINC00961 was demonstrated in BRCA, kidney chromophobe (KICH), kidney renal papillary cell carcinoma (KIRP) and LUAD. Additionally, lower RP1-78O14.1 expression was also revealed in several types of cancers including cervical squamous cell carcinoma (CESC), KIRC, KIRP and LUAD. Moreover, the significance of FENDRR down-regulation was reached in the bladder urothelial carcinoma (BLCA), colon adenocarcinoma (COAD), LUAD, Prostate adenocarcinoma (PRAD) and READ. Meanwhile, the result also showed the down-regulation of LINC01314 in cholangiocarcinoma (CHOL), esophageal carcinoma (ESCA), KICH, KIRC, KIRP, LUAD and pheochromocytoma and paraganglioma (PCPG), together with the up-regulation in the thyroid carcinoma (THCA). Interestingly, though lower expression of LINC01272 was found in LUAD, the result revealed a significant trend of up-regulation for LINC01272 in CESC, COAD, ESCA, KIRC, KIRP, READ, stomach adenocarcinoma(STAD) and uterine corpus endometrial carcinoma(UCEC). In the support of the result, GATA6-AS1 might act as a tumor suppressor in the several cancers including BLCA, CESC, ESCA, LUAD, pheochromocytoma and paraganglioma (PCPG) and UCEC. Nevertheless, MIR3945HG was only significantly lower in LUAD and there was no significant difference of LINC01572 expression between cancer tissues and para-noncancerous tissues among these 22 cancer types. All the details were presented in the Figure 11, which were derived from GEPIA.
Figure 11. Comparisons of lncRNAs expression between cancer tissues and non-cancerous tissues among 22 types of cancers involved in TCGA based on GEPIA.
(A) SFTA1P; (B) LINC00968; (C) LINC00961; (D) LINC01572; (E) RP1-78O14.1; (F) FENDRR (G) LINC01314; (H) LINC01272; (I) GATA6-AS1; (J) MIR3945HG. Y axis indicates the log2 (TPM + 1) for lncRNA expression. Green bar shows the tumor tissues and red bas indicates the non-cancerous tissues. These figures were derived from GEPIA. *: P<0.05. TPM: Transcripts per Kilobase Million.
DISCUSSION
There are marked variances in the aberrant gene profiling and molecular characteristics between LUAD and LUSC, which result in the altered therapeutic regimens administered to the two NSCLC subtypes [24–29]. Development in molecular biology has extended our awareness in decoding a wide scale of genomic unevenness that gradually leads normal lung cells to a cancerous state. In LUAD patients, EGFR-activating somatic mutations in exons 18/19/20/21 modify the sensitivity (namely exon 21 L858R, exon 19 deletion) or resistance (namely exon 20 T790M and/or insertion) to tyrosine kinase inhibitor (TKI) mediated targeted therapeutic strategies. However, as the second most frequent subtype in NSCLC, the treatment possibilities for LUSC remain very inadequate. In the current study, we focused on the aberrantly expressed lncRNAs in LUSC based on TCGA RNA-seq data. Ten lncRNAs with the highest diagnostic value (SFTA1P, LINC00968, LINC00961, LINC01572, RP1-78O14.1, FENDRR, LINC01314, LINC01272, GATA6-AS1, and MIR3945HG) were selected for further investigation of their clinical roles in LUSC. Furthermore, these lncRNAs could play essential roles in LUSC via lncRNA-mRNA networks, as well as genetic alterations, including amplification, deep deletion and mRNA upregulation.
EGFR mutations are extremely rare (<5%) in LUSC [30]; nonetheless, other genetic alterations, like overexpression and gene amplification are much common in LUSC, which play pivotal roles in the biological process and disease development of LUSC [31]. This could be explained by the use of cetuximab in the FLEX phase III studies [32], and necitumumab in the SQUIRE study [33, 34]. Except the recently approved molecular target drug nivolumab [35–39], there have been no other recommendations specifically for LUSC as approved by US Food and Drug Administration. The recent molecular advances in lncRNAs could open up a new research area for the clinical setting of LUSC.
Single lncRNA in LUSC has been studied by some groups [40–43]; however, the studies based on high throughput RNA-seq data have been rarely reported. Most recently, Liu et al [22] investigated the altered lncRNAs between LUSC and LUAD. CBioPortal was used to examine lncRNA alteration frequencies, as well as the capacity to evaluate overall survival from TCGA database. In LUSC, 624 lncRNAs were observed to gain alteration rates > 1% and 64 > 10%. Two lncRNAs, including IGF2BP2-AS1 and DGCR5 were related to better overall survival in LUSC. This study [22] focused on the genetic alteration of lncRNAs in LUSC. Similarly, Wei et al [23] also compared the lncRNA transcriptional fingerprints between LUSC and LUAD based on transcriptome analysis with TCGA and GEO. They found that there were 117 dysregulated lncRNAs in LUSC, including 56 up-regulated and 61 down-regulated lncRNAs. Among our top 10 lncRNAs, only LINC00968 was mentioned in the 117 dysregulated lncRNAs identified by Wei et al [23]. Only 16 cases of paired LUSC tissue samples were examined in the study of Wei et al [23], and this could partially explained the distinction of aberrantly expressed lncRNAs found between Wei et al [23] and our current study.
The top 10 lncRNAs (SFTA1P, LINC00968, LINC00961, LINC01572, RP1-78O14.1, FENDRR, LINC01314, LINC01272, GATA6-AS1, and MIR3945HG) had extremely high diagnostic values for LUSC, since the AUCs were all over 0.99. The differential expression levels and diagnostic potency of eight among these 10 lncRNAs could also be confirmed with independent data from GEO, which further supports the findings based on TCGA. We also performed real time RT-qPCR to verify the expression level of two lncRNAs (LINC00968 and FENDRR) with clinical sample in house. Besides, some lncRNAs may also play vital parts in the survival and progression in LUSC, which make them potential novel master regulators for LUSC. Some of these lncRNAs have been reported in other diseases. Among these 10 top aberrantly expressed lncRNAs, only the role and function of FENDRR have been well documented by several studies. FENDRR was first identified as a tissue-specific lncRNA, which was a crucial modulator of the growth of heart and body wall in mice [44]. FENDRR can bind to Proteasome component 2 (PRC2) and TrxG/MLL complexes to act as a regulator of chromatin signatures that define relevant gene activity [44]. Molecular data also suggests that FENDRR plays important part at target regulatory elements via dsDNA/RNA triplex formation, and thus directly raises PRC2 residence at these sites. FENDRR can connect epigenetic mechanisms with gene regulatory networks in embryogenesis in the mouse [45]. Furthermore, multiple knockout mouse models also unveil that FENDRR is requisite for life and brain development [46]. The clinical role and molecular mechanism of FENDRR in cancers also received much attention [47]. Decreased expression of FENDRR in infantile hemangioma was detected by both microarray analysis and qPCR [48]. Down-regulation of FENDRR was found in gastric cancer and moreover, FENDRR was closely related to the poor prognosis in gastric cancer. As for the mechanism, FENDRR can modulate the metastasis of gastric cancer cells via influencing fibronectin1 expression [49]. Most recently, high throughput microarray assay and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) were conducted to confirm that FENDRR was significantly down-regulated in human Xuanwei lung cancer (XWLC) as compared to that in para normal lung tissues [50]. In the support of this study, we speculated that down-regulation of FENDRR might play a vital role in lung cancer based on TCGA dataset and our validation based on a small size of patients by real time RT-qPCR.
SFTA1P was first mentioned by a genome-wide association (GWAS) study which investigated the susceptibility genes in the risk for dental caries. SNP rs11256676 in Phenotypes DMFS5mand of Chr. 10p14 was discovered and its function was unknown in 2013 [51]. Interestingly, SFTA1P was later reported to be predominately up-regulated in lung adenocarcinoma and one of the most remarkable enriched functions was surfactant homeostasis by array-based transcriptional survey in 2014 [52]. On the contrary, SFTA1P was found to be down-regulated in LUSC tissues in the current study, which indicates the distinct role of SFTA1P in LUAD and LUSC.
Additionally, two lncRNAs, MIR3945HG V1 and MIR3945HG V2, were identified as novel candidate diagnostic markers for tuberculosis [53]. But LINC01314, LINC00968, LINC00961, LINC01572, GATA6-AS1, RP1-78O14.1 and LINC01272 are absolutely new lncRNAs, since no publications were available by far. The clinical role of these novel lncRNAs needs further verification in LUSC.
The exact mechanisms of these aberrantly expressed lncRNAs in LUSC remain unknown. An emerging signature tune in the non-coding RNA world goes to the crosstalk between lncRNAs and mRNAs. We then predicted the prospective regulation of lncRNA co-expressed mRNA. Several lncRNAs might exert their functions via co-expressing with mRNA. Even none of WGCNA has been verified in LUSC, it is quite likely to perform in-depth studies to reveal the pathogenesis of LUSC based on aberrantly expressed lncRNAs. Furthermore, the genetic alterations can also regulate the function of certain lncRNA, and thus influence the clinical outcome [54–57]. The roles of lncRNA genetic alterations in LUSC have not been well established. Only several studies explored single lncRNAs and their genetic variants in lung cancer. For instance, among the advanced lung cancer patients, cases with rs3200401 CT and CT + TT genotypes in MALAT1 had clearly better prognosis than those with the MALAT1 rs3200401 CC genotype [58]. SNP rs114020893 of NEXN-AS1 at 1p31.1 might also contribute to lung cancer susceptibility [59].
In the current study, gene amplification, deep deletion and mRNA upregulation were detected in SFTA1P, LINC00968, LINC00961 and FENDRR and these genetic alterations of the lncRNAs showed a close correlation with survival of LUSC. However, the clinical potential of these genetic alterations needs to be confirmed with larger sample size and the exact mechanism of these genetic alterations also required in vitro and in vivo verification.
Overall, we show a signature of aberrantly expressed lncRNAs in LUSC tissues and the top 10 of them have great clinical value to act as diagnostic biomarkers, and indicators to evaluate the survival and progression of LUSC. However, other precise detecting methods, like real time RT-qPCR or FISH are required to validate the diagnostic potentials of these novel lncRNAs. Also, more in-depth experiments are necessary to explore the underlying mechanism of these lncRNAs in LUSC.
MATERIALS AND METHODS
TCGA dataset of LUSC
High throughput data of RNA-Seq diagnosed with LUSC were downloaded from TCGA on November 9, 2016 [22, 23, 60]. These RNA-seq data from Illumina HiSeq RNASeq platform included 504 LUSC and 49 adjacent non-cancerous lung tissues. Since the TCGA data were a community resource project, additional approval by the ethics committee of our hospital was not mandatory. Also, the present study adhered to the TCGA publication guidelines and data access policies.
Exploration of the aberrantly expressed lncRNAs in LUSC
The RNA-Seq data of LUSC with 60,483 mRNAs covers 7589 lncRNAs, as described by NCBI (https://www.ncbi.nlm.nih.gov/) or Ensembl (http://asia.ensembl .org/). The R language package DESeq [61, 62] was subsequently used for the calculation of aberrantly expressed lncRNAs (adjusted P<0.05 and the absolute log2 fold change >2), respectively. The lncRNAs of which expression was less than 1 in more than 10% of samples were excluded and the expression level of each lncRNA was log2 transformed for the downstream analysis.
Clinical role of the top 10 aberrantly expressed lncRNAs in LUSC
The receiver operating characteristic (ROC) curve was used to assess the diagnostic effectiveness of all aberrantly expressed lncRNAs in LUSC and the top 10 were then selected for further evaluation. All expression data were presented as the mean ± standard deviation (SD). The different expression levels of the top 10 aberrantly expressed lncRNAs between LUSC and non-cancerous lung tissues, as well as between different clinical groups were assessed by Student’s t test. Pearson correlation test (SPSS Inc., Chicago, IL, USA) was performed for the relationship between FGFR1 and each lncRNA in LUSC. The prognostic roles of these lncRNAs were examined with the Kaplan–Meier method, and the log-rank test was conducted to contradistinguish survival time. The univariate and multivariate cox analyses of these lncRNAs were also performed. A P-value < 0.05 represented statistical significance. The statistical analyses were all carried out by SPSS 22.0.
Potential molecular mechanism of the top 10 aberrantly expressed lncRNAs in LUSC
To explore the regulation network of the key lncRNAs, the co-expressed genes of those key lncRNAs were screened out by weighted gene co-expression network analysis (WGCNA) [63–65]. Finally, the lncRNA co-expression network was established based on WGCNA and finally visualized by Cytoscape software. Additionally, we also performed the GO analyses for the co-expression genes for six lncRNAs based on the Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/).
It could be assumed that the elevated expression of these lncRNAs in LUSC could be caused by genetic alterations, including amplification, deletion, or point mutations. Consequently, cBioPortal was used to summarize the possible genetic alterations for these the top 10 aberrantly expressed lncRNAs in LUSC, which were presented as OncoPrint. The clinical values of the genetic alterations were also evaluated.
Validation of the aberrant expression and clinical value of lncRNAs in LUSC based on GEO datasets
Data from Gene Expression Omnibus database (GEO, http://www.ncbi.nlm.nih.gov/geo) was used to validate the results from TCGA. Search strategy was as following: (cancer OR carcinoma OR squamous cell carcinoma OR SqCC OR SCC OR tumor OR tumor OR malignanc* OR neoplas*) AND (lung OR pulmonary OR respiratory OR respiration OR aspiration OR bronchi OR bronchioles OR alveoli OR pneumocytes OR “air way”). We only retained the original study that analyzed gene expression profiling between human LUSC tissues and normal control tissues. Independent sample T-test (SPSS 22.0 Inc., Chicago, IL, USA) was used for the statistical analysis of the differentially expressed level of these lncRNAs between LUSC and para-carcinoma lung tissues. The ROC curve analysis was used to validate the diagnostic value of the lncRNAs for LUSC patients based on GEO dataset.
Validation based on clinical samples of LUSC
To further verify the data from TCGA and GEO, we conducted real time RT-qPCR to detect the level of lncRNA LINC00968 and FENDRR with clinical LUSC samples (n=12) from the First Affiliated Hospital of Guangxi Medical University as previously reported [66–69]. The Ethical Committee of First Affiliated Hospital of Guangxi Medical University, China approved the present study. All participating patients provided informed consent and agreement for the research use of the clinical samples. GAPDH was used as internal reference with the primers as follows: Forward-5’-GCTCTCTGCTCCTCCTGTTC-3’, Reverse-5’-ACGACCAAATCCGTTGACTC-3’. The primers were listed as follows: LINC00968, Forward-5’-CCACTCCTTTAGTCGTTGTGC-3’; Reverse-5’- GGTCCCTCATTCCTATCCC-3’; FENDRR, Forward-5’- TAAAATTGCAGATCCTCCG-3’; Reverse-5’-AACGTTCGCATTGGTTTAGC-3’. Paired-samples t test was performed to compare the difference of lncRNAs between LUSC and non-cancerous lung tissues with SPSS 22.0. ROC curves were used to assess the effect of lncRNAs to discriminate the LUSC from non-cancerous lung tissue.
Analysis for the expression pattern of the lncRNAs in all tumors involved in TCGA based on GEPIA
We also showed the expression levels of the lncRNAs between cancer tissues and para-noncancerous tissues with the assistance of GEPIA (http://gepia.cancer-pku.cn), which could analyze the RNA sequencing expression data of 23 types of cancers and normal samples from the TCGA according to the standard processing pipeline.
SUPPLEMENTARY MATERIALS TABLE
Acknowledgments
The study was supported by the funds of the National Natural Science Foundation of China (NSFC81560469, NSFC81660488, and NSFC81360327), the Natural Science Foundation of Guangxi, China (2015GXNSFCA139009), Guangxi Medical University Training Program for Distinguished Young Scholars (2017), and the Guangxi Provincial Health Bureau Scientific Research Project (Z2013201). The funders had no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Tajima S, Takanashi Y, Koda K. Squamous cell carcinoma of the lung with highly proliferating fibromatosis-like stroma: a rare phenomenon. Int J Clin Exp Pathol. 2015;8:5870–5876. [PMC free article] [PubMed] [Google Scholar]
- 2.Lee E, Jin D, Lee BB, Kim Y, Han J, Shim YM, Kim DH. Negative effect of cyclin D1 overexpression on recurrence-free survival in stage II-IIIA lung adenocarcinoma and its expression modulation by vorinostat in vitro. BMC Cancer. 2015;15:982. doi: 10.1186/s12885-015-2001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang Y, Yang JC, Hsu FM, Chen YH, Shih JY, Lin ZZ, Lan KH, Cheng AL, Kuo SH. The response, outcome and toxicity of aggressive palliative thoracic radiotherapy for metastatic non-small cell lung cancer patients with controlled extrathoracic diseases. PLoS One. 2015;10:e0145936. doi: 10.1371/journal.pone.0145936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin SY, Peng F. Association of SIRT1 and HMGA1 expression in non-small cell lung cancer. Oncol Lett. 2016;11:782–788. doi: 10.3892/ol.2015.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegfried JM, Lin Y, Diergaarde B, Lin HM, Dacic S, Pennathur A, Weissfeld JL, Romkes M, Nukui T, Stabile LP. Expression of PAM50 genes in lung cancer: evidence that interactions between hormone receptors and HER2/HER3 contribute to poor outcome. Neoplasia. 2015;17:817–825. doi: 10.1016/j.neo.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An HJ, Lee YJ, Hong SA, Kim JO, Lee KY, Kim YK, Park JK, Kang JH. The prognostic role of tissue and serum MMP-1 and TIMP-1 expression in patients with non-small cell lung cancer. Pathol Res Pract. 2016;212:357–364. doi: 10.1016/j.prp.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Masuda D, Masuda R, Matsuzaki T, Imamura N, Aruga N, Tanaka M, Inokuchi S, Kijima H, Iwazaki M. Ki-67 labeling index affects tumor infiltration patterns of lung squamous cell carcinoma. Mol Med Rep. 2015;12:7303–7309. doi: 10.3892/mmr.2015.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takamochi K, Ohmiya H, Itoh M, Mogushi K, Saito T, Hara K, Mitani K, Kogo Y, Yamanaka Y, Kawai J, Hayashizaki Y, Oh S, Suzuki K, Kawaji H. Novel biomarkers that assist in accurate discrimination of squamous cell carcinoma from adenocarcinoma of the lung. BMC Cancer. 2016;16:760. doi: 10.1186/s12885-016-2792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu C, Chen H, Shan Z, Yang L. Identification of differentially expressed genes between lung adenocarcinoma and lung squamous cell carcinoma by gene expression profiling. Mol Med Rep. 2016;14:1483–1490. doi: 10.3892/mmr.2016.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang T, Li J, Zhang C, Hong Q, Jiang D, Ye M, Duan S. Distinguishing lung adenocarcinoma from lung squamous cell carcinoma by two hypomethylated and three hypermethylated genes: a meta-analysis. PLoS One. 2016;11:e0149088. doi: 10.1371/journal.pone.0149088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song DH, Ko GH, Lee JH, Lee JS, Lee GW, Kim HC, Yang JW, Heo RW, Roh GS, Han SY, Kim DC. Myoferlin expression in non-small cell lung cancer: prognostic role and correlation with VEGFR-2 expression. Oncol Lett. 2016;11:998–1006. doi: 10.3892/ol.2015.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin C, Omar M, Tunca H, Kalemci S, Ozseker B, Akbaba G, Tanriverdi O. Weight loss at the time of diagnosis is not associated with prognosis in patients with advanced-stage non-small cell lung cancer. J BUON. 2015;20:1576–1584. [PubMed] [Google Scholar]
- 13.Smardova J, Liskova K, Ravcukova B, Malcikova J, Hausnerova J, Svitakova M, Hrabalkova R, Zlamalikova L, Stano-Kozubik K, Blahakova I, Speldova J, Jarkovsky J, Smarda J. Complex analysis of the p53 tumor suppressor in lung carcinoma. Oncol Rep. 2016;35:1859–1867. doi: 10.3892/or.2015.4533. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Song P, Li H, Guo H, Jia H, Zhang B. Epidermal growth factor receptor tyrosine kinase inhibitors with conventional chemotherapy for the treatment of non-small cell lung cancer. Onco Targets Ther. 2016;9:13–20. doi: 10.2147/OTT.S94108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XW, Chen SY, Xue DW, Xu HH, Yang LH, Xu HT, Wang EH. Expression of Nemo-like kinase was increased and negatively correlated with the expression of TCF4 in lung cancers. Int J Clin Exp Pathol. 2015;8:15086–15092. [PMC free article] [PubMed] [Google Scholar]
- 16.Cao MX, Jiang YP, Tang YL, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget. 2017;8:12472–12483. doi: 10.18632/oncotarget.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrati S, De Summa S, Pilato B, Petriella D, Lacalamita R, Tommasi S, Pinto R. Next-generation sequencing: advances and applications in cancer diagnosis. Onco Targets Ther. 2016;9:7355–7365. doi: 10.2147/OTT.S99807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng H, Zhang J, Shi J, Guo Z, He C, Ding L, Tang JH, Hou Y. Role of long non-coding RNA in tumor drug resistance. Tumour Biol. 2016;37:11623–11631. doi: 10.1007/s13277-016-5125-8. [DOI] [PubMed] [Google Scholar]
- 19.Jing W, Li N, Wang Y, Liu X, Liao S, Chai H, Tu J. The prognostic significance of long noncoding RNAs in non-small cell lung cancer: a meta-analysis. Oncotarget. 2017;8:3957–3968. doi: 10.18632/oncotarget.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Hou J, Hu Z, Gu B, Shi Y. Multiple mutations of lung squamous cell carcinoma shared common mechanisms. Oncotarget. 2016;7:79629–79636. doi: 10.18632/oncotarget.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Wu Y, Yu W, Li H. Identification of a seven-miRNA signature as prognostic biomarker for lung squamous cell carcinoma. Oncotarget. 2016;7:81670–81679. doi: 10.18632/oncotarget.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Chen Y, Yang J. LncRNAs are altered in lung squamous cell carcinoma and lung adenocarcinoma. Oncotarget. 2017;8:24275–24291. doi: 10.18632/oncotarget.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Zhang X. Transcriptome analysis of distinct long non-coding RNA transcriptional fingerprints in lung adenocarcinoma and squamous cell carcinoma. Tumour Biol. 2016 doi: 10.1007/s13277-016-5422-2. [DOI] [PubMed] [Google Scholar]
- 24.Orlhac F, Soussan M, Chouahnia K, Martinod E, Buvat I. 18F-FDG PET-derived textural indices reflect tissue-specific uptake pattern in non-small cell lung cancer. PLoS One. 2015;10:e0145063. doi: 10.1371/journal.pone.0145063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, Yuan X, Qiu H, Li Q. Single-nucleotide polymorphisms of TGFbeta1 and ATM associated with radiation-induced pneumonitis: a prospective cohort study of thoracic cancer patients in China. Int J Clin Exp Med. 2015;8:16403–16413. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Shao Y, Guan B, Hao J, Cheng X, Ji K, Wang K. Extracapsular extension is a powerful prognostic factor in stage IIA-IIIA non-small cell lung cancer patients with completely resection. Int J Clin Exp Pathol. 2015;8:11268–11277. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Du Z, Li L, Shi M, Yu Y. Beclin 1 and p62 expression in non-small cell lung cancer: relation with malignant behaviors and clinical outcome. Int J Clin Exp Pathol. 2015;8:10644–10652. [PMC free article] [PubMed] [Google Scholar]
- 28.Gurel D, Ulukus C, Karacam V, Ellidokuz H, Umay C, Oztop I, Sarioglu S. The prognostic value of morphologic findings for lung squamous cell carcinoma patients. Pathol Res Pract. 2016;212:1–9. doi: 10.1016/j.prp.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, He J, Zhang D. Long noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathway. Onco Targets Ther. 2015;8:2657–2664. doi: 10.2147/OTT.S90485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 32.Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE, Paz-Ares L, Storkel S, Schumacher KM, von Heydebreck A, Celik I, O’Byrne KJ. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 33.Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Balint B, Losonczy G, Kazarnowicz A, Park K, Schumann C, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson L. Lung cancer: squiring immunotherapy to CheckMate. Nat Rev Clin Oncol. 2015;12:436. doi: 10.1038/nrclinonc.2015.110. [DOI] [PubMed] [Google Scholar]
- 35.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21:634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasan Ali O, Diem S, Markert E, Jochum W, Kerl K, French LE, Speiser DE, Fruh M, Flatz L. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology. 2016;5:e1231292. doi: 10.1080/2162402X.2016.1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, Sunami K, Kitazono S, Mizugaki H, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, et al. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol. 2016;27:2242–2250. doi: 10.1093/annonc/mdw416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, Mastromartino M, Del Bene G, Prete A, Longo F, Cortesi E, Gazzaniga P. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep. 2016;6:31726. doi: 10.1038/srep31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang YL, Lim JS, Soo RA. Profile of nivolumab in the treatment of metastatic squamous non-small-cell lung cancer. Onco Targets Ther. 2016;9:3187–3195. doi: 10.2147/OTT.S84356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao X, Zhao R, Chen Q, Zhao Y, Zhang B, Zhang Y, Yu J, Han G, Cao W, Li J, Chen X. MALAT1 might be a predictive marker of poor prognosis in patients who underwent radical resection of middle thoracic esophageal squamous cell carcinoma. Cancer Biomark. 2015;15:717–723. doi: 10.3233/CBM-150513. [DOI] [PubMed] [Google Scholar]
- 41.Luo J, Tang L, Zhang J, Ni J, Zhang HP, Zhang L, Xu JF, Zheng D. Long non-coding RNA CARLo-5 is a negative prognostic factor and exhibits tumor pro-oncogenic activity in non-small cell lung cancer. Tumour Biol. 2014;35:11541–11549. doi: 10.1007/s13277-014-2442-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Zhu N, Chen X. A novel long noncoding RNA LINC01133 is upregulated in lung squamous cell cancer and predicts survival. Tumour Biol. 2015;36:7465–7471. doi: 10.1007/s13277-015-3460-9. [DOI] [PubMed] [Google Scholar]
- 43.Saghaeian Jazi M, Samaei NM, Ghanei M, Shadmehr MB, Mowla SJ. Overexpression of the non-coding SOX2OT variants 4 and 7 in lung tumors suggests an oncogenic role in lung cancer. Tumour Biol. 2016;37:10329–10338. doi: 10.1007/s13277-016-4901-9. [DOI] [PubMed] [Google Scholar]
- 44.Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, Macura K, Blass G, Kellis M, Werber M, Herrmann BG. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grote P, Herrmann BG. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol. 2013;10:1579–1585. doi: 10.4161/rna.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, Sanchez-Gomez DB, Hacisuleyman E, Li E, Spence M, Liapis SC, Mallard W, Morse M, et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. ELife. 2013;2:e01749. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget. 2016;7:8601–8612. doi: 10.18632/oncotarget.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Lv R, Zhang L, Xu G, Bi J, Gao F, Zhang J, Xue F, Wang F, Wu Y, Fu C, Wang Q, Huo R. Long noncoding RNA expression profile of infantile hemangioma identified by microarray analysis. Tumour Biol. 2016 doi: 10.1007/s13277-016-5434-y. [DOI] [PubMed] [Google Scholar]
- 49.Xu TP, Huang MD, Xia R, Liu XX, Sun M, Yin L, Chen WM, Han L, Zhang EB, Kong R, De W, Shu YQ. Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. J Hematol Oncol. 2014;7:63. doi: 10.1186/s13045-014-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Wu C, Song G, Zhang H, Shan B, Duan Y, Wang Y. Genome-wide analysis of long noncoding rna expression profiles in human xuanwei lung cancer. Clin Lab. 2015;61:1515–1523. doi: 10.7754/clin.lab.2015.150323. [DOI] [PubMed] [Google Scholar]
- 51.Shaffer JR, Feingold E, Wang X, Lee M, Tcuenco K, Weeks DE, Weyant RJ, Crout R, McNeil DW, Marazita ML. GWAS of dental caries patterns in the permanent dentition. J Dent Res. 2013;92:38–44. doi: 10.1177/0022034512463579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao W, Luo J, Jiao S. Comprehensive characterization of cancer subtype associated long non-coding RNAs and their clinical implications. Sci Rep. 2014;4:6591. doi: 10.1038/srep06591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Yang J, Wang J, Wen Q, Wang H, He J, Hu S, He W, Du X, Liu S, Ma L. Microarray analysis of long noncoding RNA and mRNA expression profiles in human macrophages infected with Mycobacterium tuberculosis. Sci Rep. 2016;6:38963. doi: 10.1038/srep38963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, He Z, Gu Y, Fang L, Lv X. Prioritization of non-coding disease-causing variants and long non-coding RNAs in liver cancer. Oncol Lett. 2016;12:3987–3994. doi: 10.3892/ol.2016.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu H, Shang X, Shi Y, Yang Z, Zhao J, Yang M, Li Y, Xu S. Genetic variants of lncRNA HOTAIR and risk of epithelial ovarian cancer among Chinese women. Oncotarget. 2016;7:41047–41052. doi: 10.18632/oncotarget.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, Hua Y, Jin J, Wang H, Du M, Zhu L, Chu H, Zhang Z, Wang M. Association of genetic variants in lncRNA H19 with risk of colorectal cancer in a Chinese population. Oncotarget. 2016;7:25470–25477. doi: 10.18632/oncotarget.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia Z, Yan R, Duan F, Song C, Wang P, Wang K. Genetic polymorphisms in long noncoding rna h19 are associated with susceptibility to breast cancer in Chinese population. Medicine. 2016;95:e2771. doi: 10.1097/MD.0000000000002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang JZ, Xiang JJ, Wu LG, Bai YS, Chen ZW, Yin XQ, Wang Q, Guo WH, Peng Y, Guo H, Xu P. A genetic variant in long non-coding RNA MALAT1 associated with survival outcome among patients with advanced lung adenocarcinoma: a survival cohort analysis. BMC Cancer. 2017;17:167. doi: 10.1186/s12885-017-3151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan H, Liu H, Liu Z, Owzar K, Han Y, Su L, Wei Y, Hung RJ, McLaughlin J, Brhane Y, Brennan P, Bickeboeller H, Rosenberger A, et al. A novel genetic variant in long non-coding RNA gene NEXN-AS1 is associated with risk of lung cancer. Sci Rep. 2016;6:34234. doi: 10.1038/srep34234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Ruan L, Yang Y, Mei Q. Identification of crucial regulatory relationships between long non-coding RNAs and protein-coding genes in lung squamous cell carcinoma. Mol Cell Probes. 2016;30:146–152. doi: 10.1016/j.mcp.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Tang W, Liao Z, Zou Q. Which statistical significance test best detects oncomiRNAs in cancer tissues? An exploratory analysis. Oncotarget. 2016;7:85613–85623. doi: 10.18632/oncotarget.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C, Huang G, Wu Z, Xu Y, Li X, Xue Y, Zhu Y, Zhao J, Li M, Zhang J. Integrative analyses of transcriptome sequencing identify novel functional lncRNAs in esophageal squamous cell carcinoma. Oncogenesis. 2017;6:e297. doi: 10.1038/oncsis.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahn R, Gupta R, Lai K, Chopra N, Arron ST, Liao W. Network analysis of psoriasis reveals biological pathways and roles for coding and long non-coding RNAs. BMC Genomics. 2016;17:841. doi: 10.1186/s12864-016-3188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu JJ, Ren ZR, Yan JB. Identification and functional analysis of long non-coding RNAs in human and mouse early embryos based on single-cell transcriptome data. Oncotarget. 2016;7:61215–61228. doi: 10.18632/oncotarget.11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Ni H, Zhao Y, Chen K, Li M, Li C, Zhu X, Fu Q. Potential role of lncRNAs in contributing to pathogenesis of intervertebral disc degeneration based on microarray data. Med Sci Monit. 2015;21:3449–3458. doi: 10.12659/MSM.894638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Z, Huang L, Shen S, Li J, Lu H, Mo W, Dang Y, Luo D, Chen G, Feng Z. Sp1 cooperates with Sp3 to upregulate MALAT1 expression in human hepatocellular carcinoma. Oncol Rep. 2015;34:2403–2412. doi: 10.3892/or.2015.4259. [DOI] [PubMed] [Google Scholar]
- 67.Guo S, Chen W, Luo Y, Ren F, Zhong T, Rong M, Dang Y, Feng Z, Chen G. Clinical implication of long non-coding RNA NEAT1 expression in hepatocellular carcinoma patients. Int J Clin Exp Pathol. 2015;8:5395–5402. [PMC free article] [PubMed] [Google Scholar]
- 68.Pan LJ, Zhong TF, Tang RX, Li P, Dang YW, Huang SN, Chen G. Upregulation and clinicopathological significance of long non-coding NEAT1 RNA in NSCLC tissues. Asian Pac J Cancer Prev. 2015;16:2851–2855. doi: 10.7314/apjcp.2015.16.7.2851. [DOI] [PubMed] [Google Scholar]
- 69.Liu JH, Chen G, Dang YW, Li CJ, Luo DZ. Expression and prognostic significance of lncRNA MALAT1 in pancreatic cancer tissues. Asian Pac J Cancer Prev. 2014;15:2971–2977. doi: 10.7314/apjcp.2014.15.7.2971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.