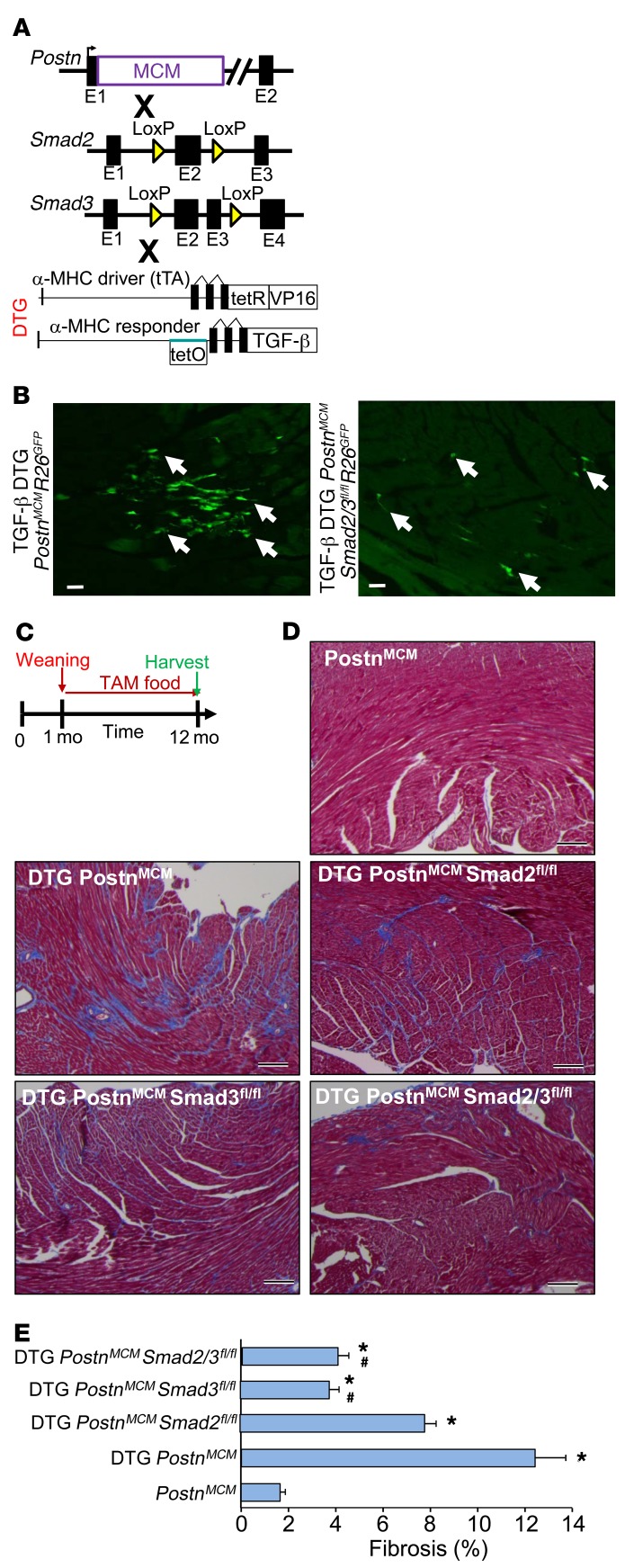
Figure 6. Fibroblast-specific deletion of Smad2/3 reduces TGF-β–induced myocardial fibrosis.
(A) Schematic representation of PostnMCM mice crossed to Smad2- and/or Smad3-loxP mice in conjunction with an inducible heart-specific latency-escaping TGF-β mutant cDNA driven by a cardiomyocyte-specific DTG system. (B) Representative histological heart sections with GFP+ interstitial cells (white arrows) showing that the TGF-β DTG effectively mediated Cre-dependent induction of PostnMCM/+activity, facilitating R26EGFP recombination and expression, while deletion of Smad2/3 reduced total EGFP+ cells. Hearts were harvested 4 weeks after tamoxifen administration. n = 4 mice harvested. Scale bars: 10 μm. (C) Experimental schematic whereby data shown in D and E were from mice fed tamoxifen through 12 months of age. (D and E) Pictures of Masson’s trichrome–stained cardiac histological sections and quantitation of the area of fibrosis (blue staining) in the indicated genotypes of mice in the presence of the TGF-β mutant transgenic system (DTG) for 12 months. Data are shown as average fibrotic area ± SEM. n = 6–8 in each group. *P < 0.05 versus PostnMCM/+; #P < 0.05 versus DTG PostnMCM/+. P values were calculated by 1-way ANOVA with post hoc Tukey’s HSD. Scale bars: 150 μm.