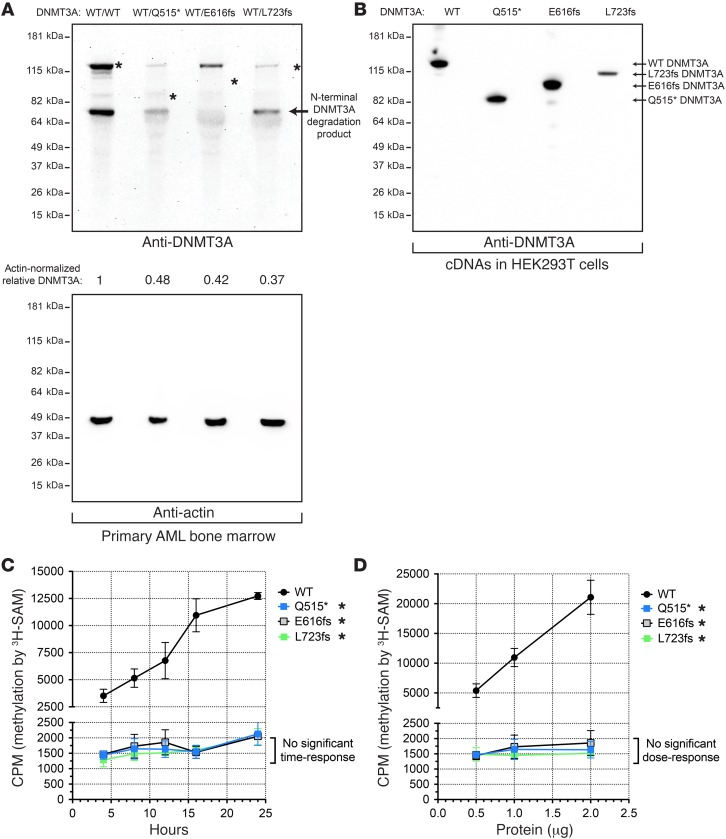
Figure 1. Truncated DNMT3A proteins are absent in AML cells, but stable in HEK293T cells, and lack de novo DNA methyltransferase activity.
(A) Western blot of endogenous DNMT3A (top panel) or actin (bottom panel) from primary AML bone marrow samples (DNMT3AWT/WT, DNMT3AWT/Q515*, DNMT3AWT/E616fs, and DNMT3AWT/L723fs). Asterisks indicate predicted positions of DNMT3A based on corresponding cDNAs (B). (B) Western blot of exogenous DNMT3A produced by WT, Q515*, E616fs, and L723fs DNMT3A cDNAs expressed in HEK293T cells. (C) In vitro methylation of a linearized plasmid DNA substrate (pcDNA3.1) by recombinant full-length human WT or mutant DNMT3A (Q515*, E616fs, or L723fs). Time-course assays using 1 μg of total protein per 35 μl reaction (250 nM). (D) In vitro methylation of a linearized plasmid DNA substrate (pcDNA3.1) by recombinant full-length human WT or mutant DNMT3A (Q515*, E616fs, or L723fs). Dose response with fixed 16-hour incubation. All experiments were independently performed 3 times, and data for C and D are shown as mean ± SEM of 3 independent experiments, each performed in triplicate. *P < 0.05, 2-way ANOVA relative to WT DNMT3A.