Figure 6.
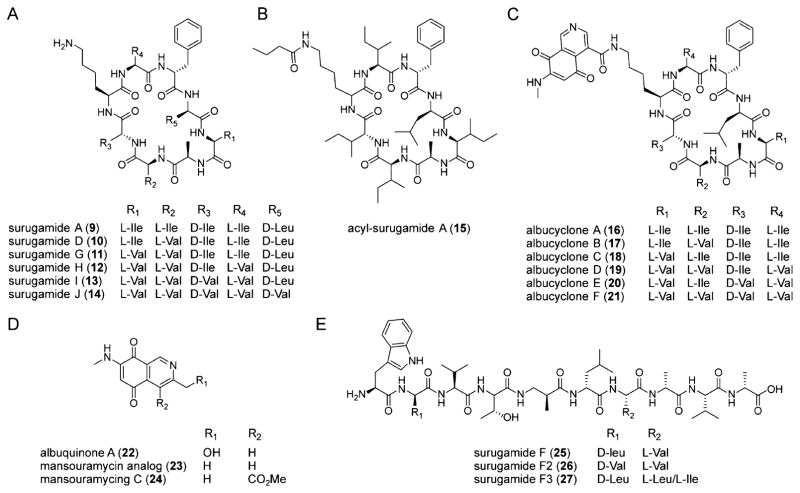
Small molecule products of the sur cluster induced by etoposide and ivermectin. (A) Structures of the surugamides. Variants A and D were previously elucidated;30 derivatives G, H, I, and J are new analogs identified in this study. (B, C) Structures of the cryptic and novel acyl-surugamide A (B) and albucyclones (C). The source of structural variability in the albucyclones is shown. (D) Structures of albuquinone A, mansouramycin C, and a mansouramycin analog. Compounds 22 and 23 are elicited by etoposide or ivermectin, and are likely produced by a separate gene locus in S. albus. (E) Structures of surugamide F and variants. Analog F was solved previously;24 variants F2 and F3 were found in this study and their proposed structures are supported by HR-MS and tandem HR-MS. Note that the stereochemistries are based on bioinformatic analyses (Figure S21) and on results from Streptomyces sp. JAMM992.24,30