Abstract
There has been very limited study of patients with chronic disease receiving potentially actionable genomic based results or the utilization of genetic counselors in the online result delivery process. We conducted a randomized controlled trial on 199 patients with chronic disease each receiving eight personalized and actionable complex disease reports online. Primary study aims were to assess the impact of in-person genomic counseling on 1) causal attribution of disease risk, 2) personal awareness of disease risk, and 3) perceived risk of developing a particular disease. Of 98 intervention arm participants (mean age = 57.8; 39% female) randomized for in-person genomic counseling, 76 (78%) were seen. In contrast, control arm participants (n=101; mean age = 58.5; 54% female) were initially not offered genomic counseling as part of the study protocol but were able to access in-person genomic counseling, if they requested it, 3-months post viewing of at least one test report and post-completion of the study-specific follow-up survey. A total of 64 intervention arm and 59 control arm participants completed follow-up survey measures. We found that participants receiving in-person genomic counseling had enhanced objective understanding of the genetic variant risk contribution for multiple complex diseases. Genomic counseling was associated with lowered participant causal beliefs in genetic influence across all eight diseases, compared to control participants. Our findings also illustrate that for the majority of diseases under study, intervention arm participants believed they knew their genetic risk status better than control arm subjects. Disease risk was modified for the majority during genomic counseling, due to the assessment of more comprehensive family history. In conclusion, for patients receiving personalized and actionable genomic results through a web portal, genomic counseling enhanced their objective understanding of the genetic variant risk contribution to multiple common diseases. These results support the development of additional genomic counseling interventions to ensure a high level of patient comprehension and improve patient-centered health outcomes.
INTRODUCTION
The ability to simultaneously analyze multiple genomic risk variants for common adult-onset disease, and apply this information in a meaningful way to patients remains a formidable challenge. Given the complexity of common health conditions like diabetes, coronary artery disease and cancer, the application of genome-based analyses can provide insight into risk but is only part of the risk equation (Eichler et al., 2010). Family health history, medical history and health behavior attributes (e.g. causal beliefs, lifestyle) must also be factored into how disease risk is presented to and perceived by the individual (Dewey et al., 2011; Inglis, Koehn, McGillivray, Stewart, & Austin, 2015; Ormond, 2013). Influences such as level of education, genetic and genomic knowledge, numeracy and literacy, and health status may affect an individual’s ability to understand, process, and incorporate genomic risk information for common adult-onset disease (Haga, 2014; Haga et al., 2013; Lautenbach, Christensen, Sparks, & Green, 2013; McBride et al., 2009; O’Neill, McBride, Alford, & Kaphingst, 2010; Roberts, Dolinoy, & Tarini, 2014).
Effective genetic counseling should make disease risk information understandable and personally relevant. To achieve these goals in the era of genomics, genetic counseling has incrementally evolved into “genomic counseling”, which takes the traditional diagnosis-focused approach for a single or few diseases, expands it to a greater number of conditions, and includes a more prevention oriented approach (Middleton, Hall, & Patch, 2015; O’Daniel, 2010; Ormond, 2013). Presenting genomic risk influences in the context of non-genetic risk variables through genomic counseling may help individuals recognize that for some diseases, the genetic contribution is more significant (e.g. age related macular degeneration), while for other diseases, lifestyle attributes, such as body mass index (e.g. type 2 diabetes) are paramount. As more genome based tests become available, it will be important to develop genomic counseling strategies for providing risk information for diseases with multiple levels of risk and complexity (Cameron, Marteau, Brown, Klein, & Sherman, 2012; Marteau & Weinman, 2006; Shelton & Whitcomb, 2015). The study of disease risk perceptions and the impact of genomic counseling on this process may increase understanding of how genomic risk information could facilitate informed decisions, aid adaptation to personal risk, and influence actions to improve health outcomes (Cameron et al., 2012; Heshka, Palleschi, Howley, Wilson, & Wells, 2008). Although studies have shown that genetic counseling can positively affect risk perception for hereditary cancer (Julian-Reynier et al., 2011; McInerney-Leo et al., 2006) and for Alzheimer disease (Ashida et al., 2010), research is needed to assess whether genetic/genomic counseling modifies risk perception for other diseases (Smerecnik, Mesters, Verweij, de Vries, & de Vries, 2009). The study of the impact of multiplex genomic testing on healthy individuals has shown perceptions of disease risk were mostly influenced by prior beliefs about genetic causality of diseases, and by family history (Shiloh et al., 2015). Little is known about how participants offered genomic counseling for multiple potentially actionable diseases perceive its potential benefit. Prior studies show that 10% or fewer individuals offered genetic/genomic counseling for genome based results received through online delivery have used this service (Bloss, Wineinger, Darst, Schork, & Topol, 2013; Kaufman, Bollinger, Dvoskin, & Scott, 2012; Schmidlen et al., 2014).
Patients with chronic disease may have different motivations for predictive testing and represent more varied socio-economic status (SES) than “healthy” individuals seeking predictive genomic risk information. Individuals with a chronic disease may also vary in their understanding and response to multiple actionable genomic risk reports, and may treat risk information related to their diagnosis differently than risk information for other diseases. The Ohio State University-Coriell Personalized Medicine Collaborative (OSU-CPMC) was designed as a randomized cohort study to measure the effects of in-person post-test genomic counseling on patients with chronic disease (heart failure; hypertension) receiving multiple personalized and potentially actionable complex disease reports through a web-based portal (Sweet et al., 2014). The primary aims of the randomized trial were to explore the following hypotheses: 1) Is genomic counseling associated with changes in causal attribution of disease risk and personal awareness of disease risk among participants with chronic disease following receipt of multiple genomic results online? 2) Does perceived risk of developing a particular disease increase among participants who receive genomic counseling? We also sought to examine the extent to which genomic counseling was associated with changes in: disease risk due to updated/expanded family history collection following assessment by the genetic counselor, genetic/genomic knowledge, and overall satisfaction.
METHODS
Background
Participants in the Coriell Personalized Medicine Collaborative (CPMC) receive multiple potentially actionable complex disease and pharmacogenomics risk reports through a secure web portal as described by Keller et al (Keller M, 2010). All CPMC participants are administered online surveys that collect demographic, medical and family histories, lifestyle, and medication information to produce personalized risk reports that are based on genetic risk factors, family history, and non-genetic risk influences (e.g. BMI). All CPMC participants also have the option to complete an online genetic education and genetic knowledge survey. The CPMC web portal also offers text and multimedia format educational materials and tools that enable study participants to learn more about basic genetics concepts, complex disease genetics, pharmacogenetics, family history risk, relative risk and health condition specific summaries detailing disease etiology, risk factors, treatment and available preventative or risk reducing actions. Results from primary outcomes of various trials related to the CPMC have been previously reported (Gordon et al., 2012; Schmidlen et al, 2016; Schmidlen et al., 2014; Sweet et al., 2016).
Study Design and Participants
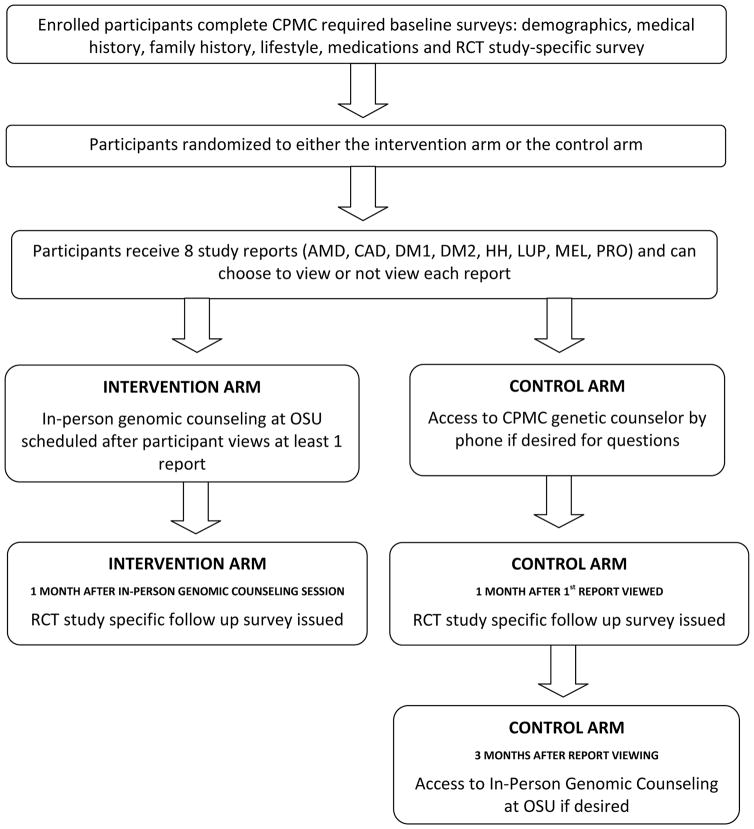
To assess the effects of in-person genomic counseling on patients with chronic disease, a separate sub-study [The Ohio State University-Coriell Personalized Medicine Collaborative (OSU-CPMC)] was conducted on a group of participants enrolled in the larger Coriell Personalized Medicine Collaborative (CPMC). The present sample is comprised of 199 out-patients with either hypertension or congestive heart failure. In addition to completing CPMC required questionnaires, OSU-CPMC participants completed baseline (76 question) and follow-up (90 question) surveys designed to measure perceived risk, causal attribution and personal awareness of disease risk; general and relative risk numeracy; genetics/genomics knowledge; confidence in use of test results, and genomic counseling satisfaction (if applicable) (Study Schema, Figure 1; Survey Questions, Table I). Additional questions (e.g. measurement of health behaviors) were also part of the OSU-CPMC study surveys and will be published separately. The study was approved by the institutional review boards at Ohio State and the Coriell Medical Institute. Informed consent was obtained from all study subjects.
Figure 1. Study Schematic.
Legend:
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
CPMC: Coriell Personalized Medicine Collaborative
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
RCT: Randomized Controlled Trial
Table I.
Study-Specific Survey Questions
| Causal Attributes of Disease Risk |
Baseline and Follow up How much do you think having a genetic risk variant determines whether or not a person will develop each of the following conditions? How much do you think family history determines whether or not a person will develop each of the following conditions? How much do you think environmental risk factors (for example, smoking, poor diet, high Body Mass Index (BMI)) determine whether or not a person will develop each of the following conditions? |
| Personal Awareness of Risk |
Baseline Do you have an increased risk for any of the following conditions due to your family history? Do you have an increased risk for any of the following conditions due to your environmental risk (for example, smoking, poor diet, high Body Mass Index (BMI))? Follow up Do you have an increased risk for any of the following conditions due to your family history? Do you have an increased risk for any of the following conditions due to your environmental risk (for example, smoking, poor diet, high Body Mass Index (BMI))? Do you have an increased risk for any of the following conditions due to a CPMC genetic risk variant? |
| Perceived Risk |
Follow up What do you think is your chance of developing each of the following diseases in your lifetime? |
| Numeracy and Genetic/Genomic Knowledge |
Expanded and General numeracy scale items1 If the chance of getting a disease is 10%, how many people out of 100 would be expected to get the disease If the chance of getting a disease is 10%, how many people out of 1000 would be expected to get the disease? Imagine that we rolled a fair, six-sided die 1,000 times. Out of 1,000 rolls, how many times do you think the die would come up even (2, 4, or 6)? In the ACME PUBLISHING SWEEPSTAKES, the chance of winning a car is 1 in 1,000. What percent of tickets to ACME PUBLISHING SWEEPSTAKES win a car? Relative Risk Numeracy - Investigator Generated Questions People without a family history of coronary artery disease have a 20% risk to develop coronary artery disease. People with a family history have a relative risk of 2.0 (they are 2 times as likely to develop coronary artery disease as those without a family history). What is the risk for someone with a family history? If a person has a genetic variant that gives a relative risk for developing type 2 diabetes of 1.3, how likely are they to develop type 2 diabetes compared to someone with no copies of that genetic variant? CPMC: Personal perception of genetic knowledge Compared to most people, how would you rate your knowledge of genetics? a. Better than most people b. About average c. Less than most people CPMC: Genetic/Genomic Knowledge2,3 It is possible to see a gene with the naked eye Healthy parents can have a child with a hereditary disease The carrier of a disease gene may be completely healthy All serious diseases are hereditary Genes are inside cells The child of a disease gene carrier is always also a carrier of the same disease A gene is a piece of DNA A gene is a part of a chromosome All body parts have all of the same genes It has been estimated that a person has about 20,000 genes A person’s race and ethnicity can affect how likely they are disease Once a genetic marker for a disorder is found in a person the disorder can be prevented or cured Only mothers can pass on genetic disorders The onset of certain diseases is due to genes, environment and lifestyle 2 Each of us has variations in our genes that make it more likely that we will get certain diseases 3 A “complex disease” is a health condition brought on by many genes and lifestyle and environment 4 A single nucleotide polymorphism or “SNiP” is a variation present in some individuals that stretches across in a large section of DNA4 OSU-CPMC Study Specific: Genetic/Genomic Knowledge If a person has a genetic marker for a disorder, the person will always get the disorder 5 People who have a genetic marker for a disease are unhealthy5 A person’s health habits can influence whether or not their genes cause disease6 |
| Individual Questions/Evaluations |
Satisfaction/Confidence in Use of Results7 The genetic counseling session was about the right length of time I needed The genetic counseling session was valuable to me The genetic counselor gave me information I needed I felt better about my health after meeting with my genetic counselor I know what to do with my results |
Source: Lipkus (2001)
Source: Jallinoja (1999)
Source: Christianson (2010)
Source: Keller (2010)
Source: Furr (1999)
Source: O’Neill (2010)
Source: DeMarco (2004)
Procedures
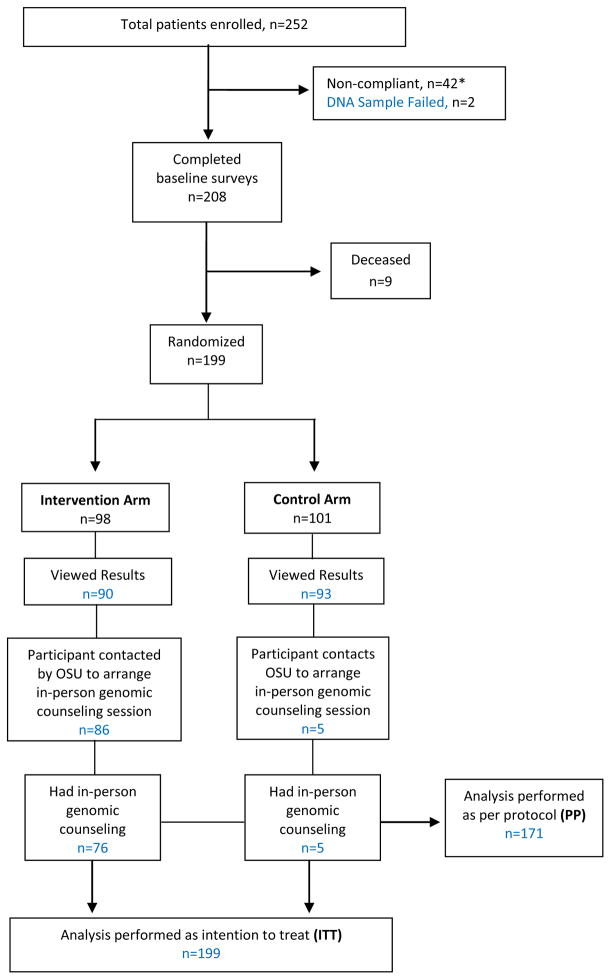
The OSU-CPMC study procedures have been described in detail previously (Sweet et al., 2014). In brief, adult patients diagnosed with either congestive heart failure or hypertension after 06/2008, and under the care of an OSU physician, were eligible for study participation. Eligible patient participants were enrolled in the clinical setting by a study recruiter who collected a saliva sample and administered a one-hour educational presentation including access to the CPMC web portal, the randomization component, background information on DNA, genes, and single nucleotide polymorphisms, CPMC test report composition, relative risk (RR), and the availability of free in-person genomic counseling. Two hundred forty-eight patients were enrolled after being identified as study eligible by OSU physicians over a two year period; 4 additional OSU patient participants were recruited via Research Match, an online NIH research registry which advertised the study Saliva samples and consent forms were sent to Coriell, and unique CPMC web portal accounts were created. Of the 252 patients enrolled, 42 were removed from the study because they failed to complete the required baseline questionnaires (Figure 2). Genotyping was unsuccessful on two patients, while an additional nine with heart failure died after completion of baseline measures but before completion of follow-up measures. Thus, of the original 252 study participants, 199 patient participants (99 heart failure, 100 hypertension) completed all required baseline evaluations.
Figure 2.
Enrollment, Study Groups, Outcomes
*Non-compliance was when an individual had not completed the baseline surveys within a 45 day time limit
Block randomization was implemented by a computer generated random number list (Microsoft SQL, Microsoft Corporation, Redmond, WA, USA) prepared by an investigator with no involvement in the trial. Participants were stratified by diagnosis (hypertension or heart failure) and enrolling physician (n=20). All 199 individuals were block randomized to either the intervention arm (98 participants) or control arm (101 participants), with each arm receiving eight CPMC personalized disease reports [age related macular degeneration (AMD), coronary artery disease (CAD), type 1 diabetes (DM1), type 2 diabetes (DM2), hemochromatosis (HH), melanoma (MEL), prostate cancer (PRO), systemic lupus erythematosus (LUP)] (Sweet et al., 2014). These eight conditions were chosen given the relative high frequency of the genetic variant used to assess risk, varied effect size of each genetic variant on risk (RR 0.08 – >6.0), and because each condition is potentially actionable via lifestyle modification and/or medical intervention. The reports present personalized risk information as relative risk for each of the 8 conditions, based on genetic variant, family history and health behavior risk factors individually, in both graphical and numeric format (Figure 3).
Figure 3.
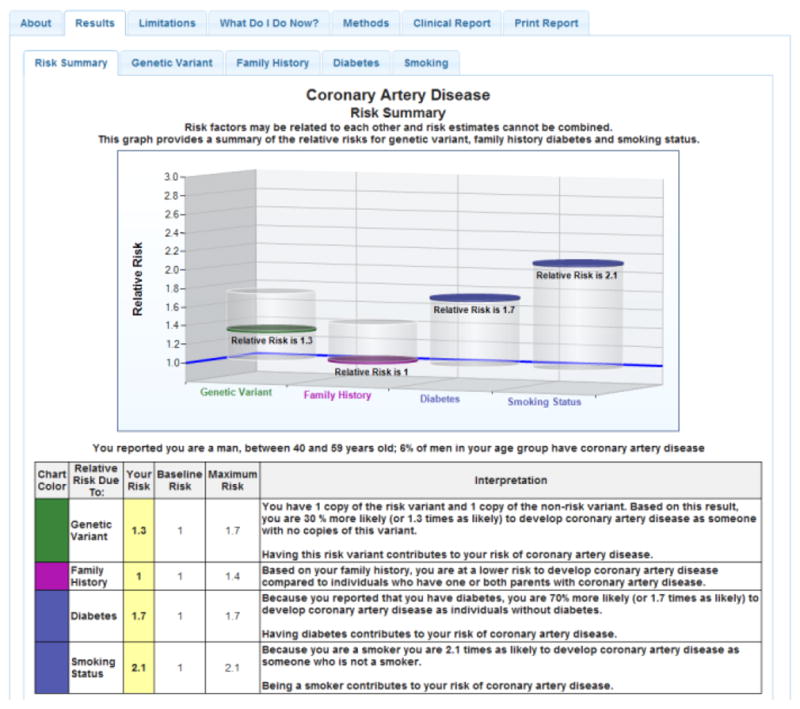
Sample CPMC Coronary Artery Disease Report
Solid discs represent the participant’s relative risk, and vertical cylinders depict the range of relative risk (RR) values possible for the risk variable. On-line risk reports are organized using a tabbed approach, with separate tabs for disease condition information, risk results, limitations, methods or review educational material. To ensure readability, the CPMC test report design was informed by multiple rounds of pilot testing conducted by allowing individuals with no scientific background to review report drafts and provide feedback.
Participants received an email notice directly from the CPMC web portal of the availability for online viewing of their test reports, and that they could choose whether or not to view each report. If a participant did not view at least one test report, study personnel contacted them by phone or email a maximum of five times over a 3-month period. When viewing a CPMC test report, participants are initially directed to a webpage containing written and video-based educational material describing the specific condition, the role of each risk factor, and approaches to prevention and treatment. Participants may choose not to view these educational materials and to proceed directly to their individual test report.
At the time of test result release, participants also received separate email notification from the Ohio State genetic counseling team reminding them of the randomization component, their assignment into either the intervention or control arm, and the availability of genomic counseling. Intervention arm participants were told they would be contacted for an in-person genomic counseling session within one month of viewing at least one of the eight CPMC reports. In contrast, control arm participants were not initially offered in-person genomic counseling as part of the study protocol but were reminded that they were able to access in-person genomic counseling, if they requested it, 3-months post viewing of at least one test report and post-completion of the follow-up survey (Figure 1). They were also reminded that they could access a CPMC genomic counselor by phone if necessary for urgent questions. The study was closed for data analysis on August 22, 2014.
Genomic Counseling Session
Genetic counseling protocols for Mendelian disorders as well as those available in the context of multiplex genomic studies were reviewed and content areas catalogued to develop the design of a structured in-person genomic counseling session (Bloss, Darst, Topol, & Schork, 2011; DeMarco, Peshkin, Mars, & Tercyak, 2004; Gollust et al., 2012; Gordon et al., 2012; Kasparian, Wakefield, & Meiser, 2007; Payne et al., 2008; Sanderson et al., 2009; Schmidlen, Gordon & Christman, 2009; Smerecnik et al., 2009; Vassy et al., 2012). The Reciprocal-Engagement Model of genetic counseling practice, built on the tenets of patient-centered education, relationship, autonomy, support provision and facilitative decision-making was used to guide the counseling process (Veach, Bartels, & Leroy, 2007). In-person genomic counseling was provided from one of two licensed genetic counselors. The genomic counseling session, which was scheduled for one hour but sometimes extended to 1.5 hours, included a review of results for all eight test reports, assessment of medical history, and, in accordance with the recommendations of the National Society of Genetic Counselors Task Force, construction of at least a 3-generation pedigree in order to provide a context in which the counselee could understand the test report risk information and risk assessment (National Society of Genetic Counselors’ Definition Task et al., 2006; Smerecnik et al., 2009). Given that participants have the potential for multiple “increased” risk variables (genetic variant, family history and health behaviors; Table SI), “decreased” risk variant(s) for DM1, and differing ranges of relative risk for each disease (0.08 – >6.0), we developed a tabular visual display for use in the genomic counseling intervention which synthesized each of the risk factors into a one-page document to provide an overall quick reference summary (Sweet et al., 2014). All individual increased risk variables were highlighted, and risk was also compared to the general population risk for each disease. The participant was asked which reports they wanted to review with the counselor, and based on this preference at least one CPMC report was accessed live via the web portal during the counseling session to associate with the quick reference summary. Genomic counseling focused on the risk factors each participant had for a given disease, to include additional disease risks identified through comprehensive review of the medical and family histories, and other health behaviors not included in the CPMC report. Specific actions to prevent and/or lower disease risk were also provided. A risk summary letter providing a focused interpretation of the eight personalized CPMC health condition study reports, the medical and family histories, recommended screening and prevention measures, and, if indicated, referral to another medical provider was then mailed to any participant that received in-person genomic counseling. The summary letter was also made available to the OSU health care team through the EPIC® electronic medical record.
Survey Measures
Baseline and follow-up measures included new items developed from a review of the literature, after review with the respective study authors, and modified items from existing CPMC surveys (Jenkins et al., 2007; Keller M, 2010; Sweet et al., 2014). Table I provides a list of questions for each of the survey measures discussed below. All survey questions included in the baseline and follow up were identical with the exception of, the follow up survey also included one question on personal awareness of disease risk based on the receipt of genetic results for each disease; one question on perceived risk of developing a particular disease; and for intervention arm participants only, 4 questions on satisfaction with the genomic counseling process.
Risk Perception
Causal Attributions of Disease Risk
A participant’s causal attribution of risk for each disease was assessed for each risk factor at baseline and follow-up (e.g. “How much do you think having a genetic risk variant determines whether or not a person will develop each of the following conditions?”) (O’Neill et al., 2010). Five point Likert scales were used for these items, and ratings were combined across diseases to generate composite scores of the overall importance a participant placed on genetic variants, family history, and environment for disease risk. Cronbach’s alphas were 0.88, 0.88, and 0.85, respectively, for these composite items.
Personal Awareness of Risk
We assessed each participant’s personal awareness of risk due to family history and environmental factors at baseline and at follow-up for each disease using original matrix format measures (“Do you have an increased risk for any of the following conditions due to your family history?”). Personal awareness of risk based on the addition of a genetic variant for each disease was then also assessed at follow-up (“Do you have an increased risk for any of the following conditions due to a CPMC genetic risk variant?”). Response options for these questions included: yes, no, not applicable, don’t know, do not want to answer.
Perceived Risk
To assess each participant’s perceived risk of developing a particular disease we used a single 5 point Likert scale validated question at follow-up only [“What do you think is your chance of developing each of the following diseases in your lifetime?”(McBride et al., 2009)]. We compared their responses to the actual risk for each disease based on the CPMC results.
Genomic Counseling Modification of Disease Risk
To examine the extent to which the genomic counseling intervention (versus control group) was associated with changes in individual actual disease risk compared to risk conveyed through the CPMC report, we recorded (1) the number of CPMC study reports for which family history or lifestyle risk was modified, (2) the number of new disease risks identified through additional medical and family history assessment, and (3) the number of specialty referrals.
Numeracy and Genetic/Genomic Knowledge
As numeracy is associated with an individual’s perceptions of risk, we adapted four numeracy scales from a previous study to assess our patient sample (Lipkus, Samsa, & Rimer, 2001). Two original multiple choice questions were included to evaluate numeracy regarding relative risk based on family history (CAD) and genetic variant (DM2), because relative risk is used in the CPMC test reports (e.g. “if a person has a genetic variant that gives a relative risk for developing type 2 diabetes of 1.3, how likely are they to develop type 2 diabetes compared to someone with no copies of that genetic variant?”) (Table I). All six numeracy questions were short answer questions scored as correct or incorrect. Personal perceptions of genetic knowledge were assessed using a single multiple-choice, original item (“Compared to most people, how would you rate your knowledge of genetics?”. Response options: better than most people; about average; less than most people).
A genetic/genomic knowledge and genetic education history survey (Table I) was used to assess knowledge of basic genetics, inheritance, influence of gene/environment interactions on complex diseases, disease susceptibility and genetic variation. The twenty questions were either from previously published studies (Christianson et al., 2010; Furr & Kelly, 1999; Jallinoja, 1999; Keller M, 2010; O’Neill et al., 2010) or formulated for the CPMC parent study to assess participant baseline genetic/genomic knowledge. Information relating to the genetic knowledge questions was covered in the participant informed consent process either as part of the explanation of the personalized medicine study provided during the consent presentation or within the text of the informed consent document. Specifically, this included an explanation of the human genome, genes, chromosomes, SNPs, complex disease genetics, and drug response. Information on the following topics was available for all participants to view on the CPMC web portal throughout the course of study: basic genetics concepts, complex disease genetics, pharmacogenetics, family history risk, and relative risk. Information relating to complex disease genetics, relative risk, lifestyle and family history risk was also provided during the in-person genomic counseling session. Knowledge was assessed using true/false questions scored as correct or incorrect. Percent correct was calculated across the sets of these questions and used as the dependent variable (follow-up) and covariate (baseline).
Individual Questions/Evaluations
Satisfaction with the content and process of the in-person genomic counseling session for intervention arm participants was assessed with 4 items from the 6 item Genetic Counseling Satisfaction Scale (GCSS)(DeMarco et al., 2004). Items included “I feel better about my health after meeting with my genetic counselor”; “The genetic counseling session was valuable to me” and “The genetic counseling session was about the right length of time I needed”. We modified one original GCSS item “My genetic counselor helped me to identify what I needed to know to make decisions about what would happen to me) to read “The genetic counselor gave me information I needed”. We replaced two original GCSS items with statements more relevant for our study (i.e. “I know what to do with my results”; “All individuals should meet with a genetic counselor when receiving this type of disease risk information”). Modifications of items from the GCSS were not validated prior to inclusion in this study. The response scale for these items was: Strongly Disagree, Disagree, Neutral, Agree, Strongly Agree, and Did Not Want to Answer.
Statistical Analyses
We used two approaches to analysis: “Per-Protocol” (PP) and “Intention to treat” (ITT) (Abraha & Montedori, 2010; Gupta, 2011). PP analysis is a comparison of the two treatment groups, and includes only those participants who completed the treatment (e.g. genomic counseling) as originally allocated. ITT analysis means that all study participants who were enrolled and randomly allocated to receive genomic counseling were included in the analysis, and are analyzed in the groups to which they were randomized. Per Protocol results are presented as the primary analyses in the main text, and ITT analyses results are included in the supplemental results for comparison. More specifically, the analyses in the main body include only individuals who completed their “treatment” (in-person genomic counseling or no in-person genomic counseling) according to the group in which they were randomized. This resulted in the removal from analyses of five participants from the randomized control group that had in-person genomic counseling prior to completing the follow-up survey. One additional individual from the control group was also removed as they received phone genomic counseling from a CPMC genetic counselor. Therefore, we had 76/98 (77.6%) of intervention arm participants receiving in-person genomic counseling; and no control arm participants (n=95) receiving in-person genomic counseling in the per protocol analysis (n=171). For socio-demographic associations (n participants=199), Student’s t-test, Fisher’s exact tests, or Wilcoxon Rank Sums tests were used as deemed appropriate.
Survey Analyses
In general, survey variables were of one of three types: 1) composite, where multiple questions are combined to get an overall score (Likert type and percent correct), 2) binary responses (yes/no, correct/incorrect), and 3) single item Likert-like questions (ordinal response 1–5), which determined the corresponding models used for their analyses. 1) For composite measurements that were summaries across multiple questions, such as percent correct across genetic knowledge questions or combined Likert scale questions, linear models were employed with a covariate for baseline scores (to adjust for baseline differences between groups) and with additional covariates for gender, age, disease group, and education level. 2) For dichotomous follow-up measurements (such as correct/incorrect assessment of self-risk), logistic models were used with covariates for baseline response, gender, age, disease group, and education level. To determine whether intervention participants had different knowledge of their personal risk compared to non-intervention participants, Fisher’s exact tests were employed. 3) For questions of certainty of personal disease occurrence (Likert type, but not Likert scale; that is, analyses of single question responses with a Likert type response), ordinal logistic models were employed with covariates for baseline response, gender, age, disease group, and education level within disease. Cronbach’s alpha (Cronbach & Warrington, 1951) was calculated for Likert type questions that together addressed a particular general theme as an indication that the combination of these questions was reliable as a Likert scale item (Clason, 1994). Missing values were assumed to be at random regarding follow-up responses. False discovery rate (Benjamini Y, 1995) adjustment was used to correct for multiple testing across main effects tests (n=86), and a false discovery rate threshold of 20% was used to declare significance.
RESULTS
Participant Socio-demographic and Clinical Characteristics
Table II and Figure 2 depict enrollment numbers and socio-demographic information for intervention and control group participants. There were no significant (p < 0.05) differences in ethnicity, gender, income or education between intervention and control group participants, or in separate analyses (PP/ITT). Sixty-nine percent (n=137) of participants had an associate’s degree or higher. There were more male participants, 107 (53.8%) than female; and 25 (12.5%) worked in a health care-related occupation (e.g. nursing). Mean age was 58.1 years (range from 24 to 94). Of the eight diseases under study, 95 participants had a personal diagnosis of at least one disease (Table SII). There were 40 subjects with 1 elevated genetic variant risk variable; 68 subjects with 2 elevated genetic risk variables; and 87 subjects with 3+ elevated genetic risk variables (Table SIII). There were no significant differences between study groups on these variables.
Table II.
Socio-demographic and Clinical Characteristics of Intervention versus Control Group Participants
| Demographic Category | Subject Category | Intervention Arm (n=76) | Control Arm (n=95) | p value | Test |
|---|---|---|---|---|---|
| Age (mean) | 57.8 | 58.5 | 0.86 | Welch’s t-test | |
| Number of reports viewed (mean) | 6.73 | 0.79 | 0.61 | Wilcoxon Rank Sums | |
| Race (Caucasian) | Yes | 68 | 88 | 0.59 | Fisher’s Exact Test |
| No | 8 | 7 | |||
| Gender | Female | 33 | 49 | 0.356 | Fisher’s Exact Test |
| Male | 43 | 46 | |||
| Education | <HS | 3 | 0 | 0.13 | Ordinal Logistic |
| HS Grad/GED | 2 | 10 | |||
| Vocational/Trade | 1 | 0 | |||
| Some College | 13 | 23 | |||
| Associate Degree | 12 | 12 | |||
| Bachelor Degree | 20 | 24 | |||
| Graduate Degree | 25 | 26 | |||
| Received GC | Yes | 76 | 0 | <0.0001 | Fisher’s Exact Test |
| No | 0 | 95 | |||
| Follow Up | Yes | 64 | 59 | 0.002 | Fisher’s Exact Test |
| No | 12 | 36 | |||
| Income | <$25k | 5 | 10 | 0.24 | Wilcoxon Rank Sums |
| $25–50k | 12 | 19 | |||
| $50–75k | 19 | 14 | |||
| $$75–100k | 19 | 17 | |||
| >$100k | 19 | 34 | |||
| Did not want to answer | 2 | 1 | |||
| Diagnosis | HTN | 43 | 44 | 0.22 | Fisher’s Exact Test |
| HF | 33 | 51 | |||
| Health Care Occupation | Yes | 9 | 13 | 0.82 | Fisher’s Exact Test |
| No | 67 | 82 |
The number of reports viewed by intervention arm participants is provided in Table III. Of 183 (91.4%) study participants who viewed at least one CPMC test report, and thus were administered the follow-up survey, 129 (64 intervention arm; 59 control arm) completed the follow-up survey. Across the entire sample, completion was greater than 90% for all follow up survey questions, with the exception of the last four questions on the survey regarding genomic counseling satisfaction. Only 29 (of 76) intervention arm participants completed these last 4 questions.
Table III.
Number of Risk Reports Viewed Per Disease By Intervention Arm Participants (n=76)
| Disease | Pre-Genomic Counseling | Post-Genomic Counseling |
|---|---|---|
| AMD | 71 | 2 |
| CAD | 64 | 4 |
| DM1 | 54 | 5 |
| DM2 | 55 | 5 |
| HH | 59 | 6 |
| LUP | 54 | 8 |
| MEL | 53 | 10 |
| PRO | 45 | 10 |
Legend:
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
Of 98 intervention arm participants, 76 (77.6%) were seen for in-person genomic counseling. Four individuals did not show up for their appointment. Of the remaining 18 eligible intervention arm participants, eight never viewed a CPMC report; six never scheduled an appointment; and four declined genomic counseling. The mean number of days from participant completion of the baseline survey to genomic counseling was 224; from release of test results to genomic counseling (90); from viewing test results to genomic counseling (52), and from genomic counseling to completion of follow-up survey (168) (Table SIV). The mean number of days from report viewing to completion of follow-up surveys was comparable between groups (intervention, 222; control, 175).
Risk Perception
Causal Attributions of Disease Risk
We examined to what extent intervention arm participants who had genomic counseling believe that different risk influences (genetic variant, family history, health behavior) contribute to a person’s risk for developing each of the eight diseases, and if receiving genomic counseling was associated with changes, from baseline in their causal attributes. At baseline, there was no evidence supporting differences in causal beliefs between the two groups. In follow-up, genomic counseling was associated with decreased genetic causal beliefs across all eight diseases, compared to control participants (estimate=0.4, raw p=0.019; FDR p=0.142, 95% C.I. 0.06–0.7); Table IV; SV ITT). Here and throughout the results, estimate refers to the estimate of the coefficient for the variable of interest in the statistical models. Additional analyses were then performed for each disease to determine if only a subset of the diseases might be driving the association. As seen in Table IV (SV, ITT), genomic counseling was positively associated with lowering participants’ causal beliefs in the degree of genetic variant influence for three diseases (LUP, raw p=0.0008, DM1, raw p=0.010; PRO, raw p=0.005; estimates = 1.3, 0.92, 1.0, respectively).
Table IV.
Causal Attributes of Genetic Variant, Family History, and Environmental Disease Risk: General Composite Score Across All Eight Diseases Under Study
| Estimate | CI (lower) | CI (upper) | Std. Error | p value | FDR p value | |
|---|---|---|---|---|---|---|
| Genetic Variant | 0.378 | 0.06 | 0.69 | 0.158 | 0.019 | 0.142 |
| Family History | 0.089 | −0.181 | 0.358 | 0.136 | 0.515 | 0.744 |
| Environmental | 0.096 | −0.18 | 0.37 | 0.139 | 0.494 | 0.744 |
| Additional Analyses: Causal Attributes of Genetic Variant Disease Risk | ||||
|---|---|---|---|---|
| Disease | Risk Factor | Estimate | Std. Error | p Value |
| AMD | Genetic Variant | 0.297 | 0.349 | 0.395 |
| CAD | Genetic Variant | 0.369 | 0.352 | 0.295 |
| DM1 | Genetic Variant | 0.923 | 0.358 | 0.010 |
| DM2 | Genetic Variant | 0.108 | 0.344 | 0.752 |
| HH | Genetic Variant | 1.101 | 0.373 | 0.003 |
| LUP | Genetic Variant | 1.274 | 0.379 | 0.0008 |
| MEL | Genetic Variant | 0.574 | 0.350 | 0.101 |
| PRO | Genetic Variant | 1.03 | 0.365 | 0.005 |
Legend:
Survey Question: How much do you think having a genetic risk variant determines whether or not a person will develop each of the following conditions?
Null Hypothesis: Genomic counseling does not influence the importance that participants place on a given factor (genetic variant, family history, environment) on determining disease risk
Estimate: The estimated coefficient in the model for Intervention Arm participants. The range of possible values is −infinity to +infinity
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
FDR: False discovery rate
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
Personal Awareness of Risk
Baseline personal awareness of risk, based on two factors, family history and health behavior, was in general accurate and highly predictive of follow up awareness of risk for each disease in each group. Upon examining whether genomic counseling affected personal awareness of risk (whether subjects correctly reported that they were at increased risk based on family history and health behavior risk influences), there was no significant effect of genomic counseling (FDR p > 0.25) Table SVI). However, we also asked participants in their follow-up questionnaire whether they knew they had an increase in disease risk due to a genetic risk variant, and found that intervention arm participants who had genomic counseling answered “don’t know” at a lower rate than control subjects, for six of the eight diseases (FDR p < 0.2; Table V; SVII ITT). We then compared the accuracy of participants’ personal awareness of disease risk to their actual genetic variant results; significant associations with genomic counseling were seen for DM1 and DM2 (FDR p=0.2 and 0.12, respectively) with more individuals in the genomic counseling arm accurately describing the risk for DM1 and DM2 than in the control arm.
Table V.
Participant Awareness of an Increase in Disease Risk Due to a Genetic Variant
| Yes n | No n | FET p value | FDR p value | |
|---|---|---|---|---|
| AMD | ||||
| GC intervention | 34 | 23 | 0.049 | 0.241 |
| Control | 9 | 35 | ||
| CAD | ||||
| GC intervention | 36 | 24 | 0.02 | 0.142 |
| Control | 27 | 18 | ||
| DM1 | ||||
| GC intervention | 12 | 48 | 0.003 | 0.050 |
| Control | 10 | 33 | ||
| DM2 | ||||
| GC intervention | 34 | 26 | 0.006 | 0.082 |
| Control | 14 | 30 | ||
| HH | ||||
| GC intervention | 9 | 45 | 0.17 | 0.453 |
| Control | 4 | 39 | ||
| LUP | ||||
| GC intervention | 17 | 40 | 0.017 | 0.142 |
| Control | 6 | 36 | ||
| MEL | ||||
| GC intervention | 19 | 41 | 0.002 | 0.04 |
| Control | 6 | 36 | ||
| PRO | ||||
| GC intervention | 5 | 47 | 0.037 | 0.197 |
| Control | 2 | 33 |
Legend:
Survey Question: Do you have an increased risk for any of the following conditions due to a CPMC genetic risk variant?
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
FDR: False Discovery Rate
FET: Fisher’s Exact Test
GC: Genomic Counseling
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
Perceived Risk
Lastly, at follow-up, we asked participants to report their perceived risk for developing each of the eight diseases in their lifetime, and compared those results to their actual risk results. Although we found no main effect due to genomic counseling, participants who had elevated BMI as a risk factor for DM2 (FDR=0.04), genetic variant risk for DM2 (FDR p=0.12; estimate=2.0; raw p=0.001), family history risk for MEL (FDR p=0.05, estimate=1.9; raw p=0.003) or genetic variant risk for MEL (FDR p=0.01, estimate=2.3, raw p=0.0002) had an elevated perceived risk for developing these diseases compared to those without these risk factors (Table VI; SVIII ITT).
Table VI.
Perceived Risk
| Disease | Risk Factor | Genomic Counseling p Value | Actual Risk p Value | Genomic Counseling FDR p Value | Actual Risk FDR p Value |
|---|---|---|---|---|---|
| AMD | Family | 0.127 | 0.270 | 0.372 | 0.574 |
| Smoking | 0.195 | 0.968 | 0.503 | 1.00 | |
| Variant | 0.082 | 0.814 | 0.307 | 0.891 | |
| CAD | Diabetes | 0.576 | 0.506 | 0.744 | 0.744 |
| Family | 0.234 | 0.565 | 0.569 | 0.744 | |
| Smoking | 0.296 | 0.564 | 0.600 | 0.744 | |
| Variant | 0.649 | 0.071 | 0.811 | 0.300 | |
| DM1 | Family | 0.101 | 0.747 | 0.334 | 0.882 |
| Variant | 0.043 | 0.492 | 0.228 | 0.744 | |
| DM2 | BMI | 0.467 | 0.001 | 0.744 | 0.043 |
| Family | 0.715 | 0.067 | 0.868 | 0.300 | |
| Variant | 0.813 | 0.010 | 0.891 | 0.119 | |
| LUP | Family | 0.067 | 0.032 | 0.300 | 0.196 |
| Variant | 0.109 | 0.102 | 0.346 | 0.333 | |
| MEL | Family | 0.249 | 0.003 | 0.572 | 0.051 |
| Variant | 0.077 | 0.0001 | 0.307 | 0.010 |
Legend:
Survey Question: What do you think is your chance of developing each of the following diseases in your lifetime?
Null hypothesis: genomic counseling and actual risk do not have an influence on a participant’s belief that they will/will not develop the disease
Hemochromatosis (HH) was not included because there were no participants with HFE mutation (the only reported risk factor) that completed follow up.
Prostate cancer (PRO) was not included because the number of participants with a risk factor was too small to estimate using modeling
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
FDR: False Discovery Rate
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
Genomic Counseling Modification of Disease Risk
To examine whether genomic counseling was associated with changes in individual actual disease risk, we compared the number of CPMC study reports for which family history risk was modified based on the collection of a three generation pedigree, as well as the number of new disease risks identified through additional family history assessment. We also recorded the number of specialty referrals that were made by this additional risk assessment. Among all study participants (intervention and control arm) who had genomic counseling (n=81), family history disease risk was modified for 61 (75.3%; 95% CI 65.9%–84.7%). There were 104 instances of specific modification of participant disease risk, which accounted for 6% of all risk variables for which individuals had a risk determined separately at genomic counseling (Table VII). Genomic counseling also identified 31 individuals who were referred for additional genetics evaluation (n=22) or increased screening (n=9) (Table VIII).
Table VII.
Genomic Counseling Modification of Disease Risk Due to Additional Family History Assessment
| Disease Variable | Test Report Risk | Post Genomic Counseling Risk | Number of Changes to Risk | Change Due to Additional Family History Obtained | Change Due To Modification of Family History Risk |
|---|---|---|---|---|---|
| AMD | No Risk | Increased | 5 | 5 | |
| AMD | Increased | No Risk | 2 | 2 | |
| Subtotal | 7 | 5 | 2 | ||
| CAD | No Risk | Increased | 11 | 11 | |
| CAD | Increased | Moderate* | 18 | 18 | |
| CAD | Increased | High** | 7 | 7 | |
| Subtotal | 36 | 36 | - | ||
| DM1 | No Risk | Increased | 1 | 1 | |
| DM1 | Increased | No Risk | 20 | 20 | |
| Subtotal | 21 | 1 | 20 | ||
| DM2 | No Risk | Increased | 13 | 13 | |
| Subtotal | 13 | 13 | - | ||
| LUP | Increased | No Risk | 23 | 23 | |
| Subtotal | 23 | - | 23 | ||
| MEL | No Risk | Increased | 2 | 2 | |
| MEL | Increased | No Risk | 2 | 2 | |
| Subtotal | 4 | 2 | 2 | ||
| TOTAL | 104 | 57 | 47 |
Legend:
Moderate: personal or family history of coronary heart disease conferring relative risk of 2.01
High: personal or family history suggestive of familial coronary heart disease generally associated with an increased risk (2–5 fold) with risk increasing based on the number of affected relatives, and early age of diagnosis2
Source: Scheuner (2003)
Source: Scheuner (2010)
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
Table VIII.
Indication for Specialty Referral
| Specialty Area | Indication | Number of Cases |
|---|---|---|
| Cardiovascular Genetics | ||
| Cardiomyopathy | 9 | |
| Familial Hypercholesterolemia | 3 | |
| Aortic Aneurysm | 1 | |
| Cancer Genetics | ||
| Hereditary breast-ovarian cancer | 5 | |
| Hereditary colorectal cancer | 1 | |
| Other hereditary cancer | 2 | |
| Medical Genetics | ||
| Muscular Dystrophy | 1 | |
| Subtotal | 22 | |
| High Risk Cardiovascular Screening clinic | 6 | |
| Inherited Arrhythmia Clinic | 2 | |
| Nutritional Services | 1 | |
| Subtotal | 9 | |
| Total | 31 |
Numeracy and Genetic/Genomic Knowledge
We used a number of questions to assess participant’s simple genetic knowledge, complex genomic knowledge, and levels of numeracy. At baseline, the mean percentage of correct answers on the 14 simple genetic knowledge questions was relatively high (77%), as well as for the six complex disease questions (75%). Basic genetic knowledge (at baseline) was associated in the multivariable model with higher levels of education (raw p <0.0001; estimate= 0.04; 95% CI 0.02–0.05). Furthermore, there was strong evidence that baseline performance was highly predictive of follow-up performance on the numeracy (Tables SIX, SX) and genetic/genomic knowledge questions (numeracy raw p< 0.0001, estimate=0.53, 95% CI 0.40–0.66; simple genetic knowledge raw p< 0.0001, estimate=0.54, 95% CI 0.34–0.74; complex genetic knowledge raw p<0.0001, estimate=0.45, 95% CI= 0.26–0.65) suggesting that in a generally highly educated group, genomic counseling did not provide an added knowledge benefit (FDR p > 0.39) (Tables SXI-SXIII), at least at the difficulty level of the questions included in the current study.
Individual Questions/Evaluations
Of intervention arm participants who had in-person genomic counseling, 83.1% (95% CI 70.5–91.2%) expressed confidence in knowing what to do with test results, as compared to control arm participants (61.8%; 95% CI 47.7%–74.3%). Likewise more intervention arm participants who received genomic counseling (73%; 95% CI 59.5%–83.3%) felt that individuals should meet with a genetic counselor when receiving these types of test results, than control arm participants (54.4%; 95% CI 40.6%–67.8%). Only 29 participants who had in-person genomic counseling completed the 4-question survey section on satisfaction; of these, 24 (82.7%; 95% CI 63.5–93.4%) reported feeling better about their health following genomic counseling. Twenty-seven participants (93%; 95% CI 75.8–98.8%) felt the genomic counseling session was the appropriate length of time. A similar percentage of counselees (93%; 95% CI 75.8–98.8%) felt the genomic counseling session was valuable, and that the counselor provided needed information (Table SXIV).
DISCUSSION
In a population of patients affected with chronic disease receiving multiple, actionable and personalized complex disease risk reports through an online portal, we sought to determine if in-person genomic counseling had an impact on 1) causal attribution of disease risk, 2) personal awareness of disease risk, and 3) perceived risk of developing a particular disease. We found that those receiving genomic counseling had enhanced objective understanding of the genetic variant risk contribution for multiple actionable complex disease reports. Indeed, participants receiving genomic counseling were significantly more likely to understand the relative and limited predictive contribution of common genetic risk factors for complex disease compared to control subjects. Participants receiving genomic counseling also were more confident and accurate in knowing their genetic risk status than control subjects, which is consistent with broader literature on the benefits of genetic counseling (Armstrong et al., 2015). Furthermore, the more comprehensive assessment of family history through genomic counseling allowed for disease risk to be modified in a significant percentage of cases. Our study participants demonstrated similarly high levels of genetic knowledge to that reported in the larger CPMC cohort (Schmidlen et al., 2016) as well as that found by Haga et al., who also studied genetic knowledge in the context of common, complex diseases (Haga et al., 2013). In a highly educated population of patients provided with genetics/genomics education during recruitment and with access to online genetics/genomics educational material prior to genomic counseling, we did not find significant improvement in genetics/genomics knowledge or numeracy following genomic counseling. Given that the information assessed by the genetic knowledge questionnaire was covered at multiple points during the study recruiting session, discussed in the informed consent document, and included in the educational web pages on the CPMC web portal, in addition to the highly educated population, these findings are not surprising. While some of the topics covered in the genetic knowledge questionnaire (complex disease genetics, family history risk) were also reinforced in the genomic counseling session, the focus of the genomic counseling sessions was on personal risk assessment and not on a review of the specific genetic/genomic knowledge items queried.
Previous studies have shown that 1) individuals see distinct causal roles for genetic variant and health behavior risk influences for common disease; and 2) these separate causal beliefs are not incompatible (Kaphingst et al., 2012; McBride et al., 2009; McBride, Birmingham, & Kinney, 2015; Sanderson et al., 2009; Shiloh et al., 2015). In fact, for common disease risk, although individuals may view separate influences on distinct tracks, large segments of the population also appear to have disparate views of the relationship between genes and health behavior attributes on perceived risk (Ashida et al., 2011; Condit & Shen, 2011; Haukkala et al., 2015). O’Neill et al. (2010) found that healthy individuals, when provided complex disease results, had a tendency to favor genetic causation over health behaviors when the number of personal risk factors increased, which corresponds with our own findings. Our sample of patients appeared to embrace stronger causal belief in the genetic influence on common disease risk, possibly due to having personal experience in dealing with a chronic disease. The genomic counseling intervention, in addition to providing more insight into the interrelationship between genetics and health behaviors as contributors of risk, may also have countered existing causal genetic deterministic beliefs and emotions predicated by personal disease experience. This may allow, in turn, greater understanding of the multifactorial nature of complex disease and an opportunity for additional interventions to improve patient-centered health outcomes (Austin, 2015; Lewis et al., 2015; McBride et al., 2015; Ormond, 2013). As all study participants received pre-test education during the informed consent session, and had access to online educational resources, genomic counseling may also have served to reinforce the test report message, and increase confidence in use of multi-page, detailed results. These findings are consistent with previous work showing that incorporation of evidence-based communication strategies in the result delivery process result in more accurate interpretation (Birch, 2015; Haga et al., 2014).
As compared to online family history collection and risk assessment, a three or four generation, more comprehensive family history was obtained and assessed through genomic counseling. In this targeted disease population, the assessment of comprehensive family history resulted in a significant number of modifications of participant disease risk, to include identification of individuals for which more targeted testing or screening was appropriate. For example, the family history relative risk value chosen by the CPMC for use in the CAD risk report came from a publication that provided family history risk assessment for CAD based only on parental history of CAD (Myers, Kiely, Cupples, & Kannel, 1990). This points to a limitation of online familial risk assessments in general, which are based on what the participant provides, but also what algorithm(s) the online tool includes, which can often be limited, incomplete or incorrect. Via genomic counseling, participants with possible Mendelian conditions were also identified. The CPMC family history risks were not designed with Mendelian disease risk detection in mind, but were designed very specifically to the complex diseases included, and usually based on first-degree relative information. While web tools can be invaluable for the purposes of triage (Sweet, Sturm, Rettig, McElroy, & Agnese, 2015), they can also miss the intricacies of a 3–4 generation pedigree assessment collected and assessed during genomic counseling. Comprehensive risk assessment by a genetic counselor, whether by interpreting medical and family histories or by incorporating genetic variant and health behavior attributes into the analysis, remains an integral part of the result delivery process in genetic/genomic counseling (National Society of Genetic Counselors’ Definition Task et al., 2006; Smerecnik et al., 2009).
Study Limitations
Unlike use of multiplex testing by “healthy” adults, our participants were all included due to their diagnosis of a chronic disease (heart failure or hypertension). We had a number of individuals who never completed baseline measures, did not view a single test report, or complete follow-up surveys. The randomized groups did not fully capture the magnitude of the genomic counseling vs. no-genomic counseling effects because some intervention group individuals did not receive genomic counseling, and some control arm subjects received genomic counseling. Our recruitment efforts may have bias, especially for the heart failure cohort, as almost 40% of eligible participants approached did not have access to a computer and thus declined participation. We had a higher than expected patient SES, and the sample was highly educated and predominantly Caucasian. There were self-reported data (e.g. family history) for the web portal, potentially introducing reporting bias. We had no control over which CPMC reports participants selected to view on their own via the web portal, with the exception of reports reviewed during the genomic counseling session. During the genomic counseling session, all eight health condition reports were reviewed with the participant. Some participants may have gotten more information than they wanted or had an interest in learning. We had no ability to track which educational topics were viewed by participants on the CPMC web portal during the course of study. While using only two genetic counselors for the in-person genomic counseling sessions helped to standardize the intervention, this limits generalizability of study findings. We utilized portions of published measures, with modification of some items, and creation of new survey measures. The low response rate for items evaluating genomic counseling received raise caution about the generalizability of these results. Given the modest sample size, which was not representative of any particular disease population, and which may have been underpowered to detect real differences, these should be considered preliminary results and further investigation is needed.
Research Recommendations and Practice Implications
Based on the limitations of this study, further research on the effects of genetic/genomic counseling on patients receiving multiple, actionable complex disease results in an online format is necessary. Given the steady increase in the availability of genomic based results, including those available through online formats, there remains appreciable need for additional research on the effectiveness and extension of genetic/genomic counseling service delivery beyond traditional referral reasons (i.e. Mendelian disease risk) and service delivery approaches (Haga et al., 2014; Lewis et al., 2015; Ormond, 2013; Shiloh et al., 2015; Trepanier & Allain, 2014). These include phone (telemedicine) as well as use of e-learning approaches (both static and interactive) either alone or to supplement counseling (Birch, 2015; Haga et al., 2014). Use of adjunct e-learning approaches and automated family history risk assessment tools may be an avenue to impact patient knowledge and improve patient-centered health outcomes while increasing the efficiency of genomic counseling interventions. The degree of genomic counseling needed will vary per patient, and per indication. In fact, counseling for common risk variants may not always require advanced or specialized counseling from a genetic counselor, but rather other health care professionals, with supplemental training in genetics/genomics (e.g. nurses) could help in this manner (Mills & Haga, 2014; O’Daniel, 2010; Ormond, 2013; Shelton & Whitcomb, 2015).
CONCLUSION
In conclusion, our findings show that genomic counseling significantly affected comprehension of the genetic variant risk contribution when patients were presented with multiple potentially actionable complex disease reports through an online portal. Our study demonstrates that genetic counselors can work in many ways to affect patient’s understanding of risk including: 1) providing appropriate breakdown of the various components of disease risk (genetic variant(s), family history, non-genetic influences) when presenting risk for multiple diseases at the same time, 2) adding additional context to this risk based on personal and family history, to include comprehensive assessment through development of a 3–4 generation pedigree, and 3) increasing patient understanding by providing side-by-side comparison of risks factors found in online test reports, to that provided in a visual one-page summary that was used in the counseling session. Our findings also suggest that genomic counseling for common disease risks, especially in the setting of patients with chronic disease receiving test results with actionable components, may allow opportunity for additional patient-centered interventions. Providing insight on the varied effect of genetic variants on risk, to include the limited predictive contribution of many of these variants, and as relative to other risk factors, may allow patients to develop more accurate perceptions of risk and what risks they can modify. Given that most common diseases are multifactorial in nature, with potentially actionable components via lifestyle modification and/or medical intervention, improving patients risk perceptions may impact personal utility and efficacy, especially if supplemented with effective health behavior recommendations and interventions.
Supplementary Material
Table SI. Reported Disease Risk Values for a Caucasian OSU-CPMC Participant
Table SII. Self-Reported Diagnoses of Study Participants
Table SIII. Number of Participants with at Least One Risk Factor
Table SIV. Number of Days Between Survey Completion, Report Release, Report Viewing and Genomic Counseling
Table SV. Causal Attributes of Genetic Variant, Family History, and Environmental Disease Risk: General Composite Score Across All Eight Diseases Under Study (Intention to Treat)
Table SVI. Baseline Personal Awareness of Family History and Environmental Risk Factors
Table SVII. Participant Awareness of an Increase in Disease Risk Due to a Genetic Variant (Intention to Treat)
Table SVIII. Perceived Risk (Intention to Treat)
Table SIX. Response to Numeracy Questions at Baseline and Follow up
Table SX. General and Relative Risk Numeracy, Combined Question Response
Table SXI. Personal Rating of Genetic Knowledge
Table SXII. Complex and Simple Genetic/Genomic Knowledge Questions
Table SXIII. Follow up (Complex/Simple) Genetic/Genomic Knowledge Adjusted for Covariates (including Numeracy)
Table SXIV. Confidence in Use of Results, Genomic Counseling Satisfaction
Acknowledgments
We thank Ray Hershberger, Albert de la Chapelle, Clay Marsh, Wolfgang Sadee, Philip Binkley, Naraj Tayal, Randy Wexler, Mary Jo Welker, Karen Wernke, Samantha DeMarsh, Sarah Adelsperger, Jennifer Lehman, Sarah Mazzola, Regina Dudzik, Amy Ehrlich, Sofia Durrani and Meagan Kane for their kind assistance with this study. We are grateful to the anonymous reviewers for their detailed comments on the original manuscript.
Funding: Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number R21HG006575. This work was also supported in part by a sub-award from the National Center for Advancing Translational Sciences via Award Number UL1TR001070; and the Ohio State University Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Center for Advancing Translational Sciences. The Coriell Personalized Medicine Collaborative was funded by the William G. Rohrer Foundation, the RNR Foundation and a grant from the endowment of the Coriell Institute for Medical Research.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: EG is currently a paid employee of Genome Medical. She worked for the Coriell Institute for Medical Research at the time that this study was developed and the majority of the data collection period. The authors have no additional conflicts of interest to disclose.
Informed Consent: All procedures followed were in accordance with the ethical standards of the local medical ethical boards of the Ohio State University Wexner Medical Center and the Coriell Institute for Medical Research and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Animal Studies: This article does not contain any studies with animals performed by any of the authors.
References
- Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ. 2010;340:c2697. doi: 10.1136/bmj.c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J, Toscano M, Kotchko N, Friedman S, Schwartz MD, Virgo KS, … Sutphen R. Utilization and Outcomes of BRCA Genetic Testing and Counseling in a National Commercially Insured Population: The ABOUT Study. JAMA Oncol. 2015:1–10. doi: 10.1001/jamaoncol.2015.3048. [DOI] [PubMed] [Google Scholar]
- Ashida S, Goodman M, Pandya C, Koehly LM, Lachance C, Stafford J, Kaphingst KA. Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics. 2011;14(4–5):307–316. doi: 10.1159/000316234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida S, Koehly LM, Roberts JS, Chen CA, Hiraki S, Green RC. The role of disease perceptions and results sharing in psychological adaptation after genetic susceptibility testing: the REVEAL Study. Eur J Hum Genet. 2010;18(12):1296–1301. doi: 10.1038/ejhg.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J. The effect of genetic test-based risk information on behavioral outcomes: A critical examination of failed trials and a call to action. Am J Med Genet A. 2015;167(12):2913–2915. doi: 10.1002/ajmg.a.37289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, HY Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Birch PH. Interactive e-counselling for genetics pre-test decisions: where are we now? Clin Genet. 2015;87(3):209–217. doi: 10.1111/cge.12430. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Darst BF, Topol EJ, Schork NJ. Direct-to-consumer personalized genomic testing. Hum Mol Genet. 2011;20(R2):R132–141. doi: 10.1093/hmg/ddr349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS, Wineinger NE, Darst BF, Schork NJ, Topol EJ. Impact of direct-to-consumer genomic testing at long term follow-up. J Med Genet. 2013;50(6):393–400. doi: 10.1136/jmedgenet-2012-101207. [DOI] [PubMed] [Google Scholar]
- Cameron LD, Marteau TM, Brown PM, Klein WM, Sherman KA. Communication strategies for enhancing understanding of the behavioral implications of genetic and biomarker tests for disease risk: the role of coherence. J Behav Med. 2012;35(3):286–298. doi: 10.1007/s10865-011-9361-5. [DOI] [PubMed] [Google Scholar]
- Christianson CA, Powell KP, Hahn SE, Bartz D, Roxbury T, Blanton SH, … Henrich VC. Findings from a community education needs assessment to facilitate the integration of genomic medicine into primary care. Genet Med. 2010;12(9):587–593. doi: 10.1097/GIM.0b013e3181ed3f97. [DOI] [PubMed] [Google Scholar]
- Clason DL, Dormody TJ. Analyzing data measured by individual Likert-type items. Journal of Agricultural Education. 1994;35(4):31–35. [Google Scholar]
- Condit CM, Shen L. Public understanding of risks from gene-environment interaction in common diseases: implications for public communications. Public Health Genomics. 2011;14(2):115–124. doi: 10.1159/000314915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronbach LJ, Warrington WG. Time-limit tests: estimating their reliability and degree of speeding. Psychometrika. 1951;16(2):167–188. doi: 10.1007/BF02289113. [DOI] [PubMed] [Google Scholar]
- DeMarco TA, Peshkin BN, Mars BD, Tercyak KP. Patient satisfaction with cancer genetic counseling: a psychometric analysis of the Genetic Counseling Satisfaction Scale. J Genet Couns. 2004;13(4):293–304. doi: 10.1023/b:jogc.0000035523.96133.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey FE, Chen R, Cordero SP, Ormond KE, Caleshu C, Karczewski KJ, … Ashley EA. Phased whole-genome genetic risk in a family quartet using a major allele reference sequence. PLoS Genet. 2011;7(9):e1002280. doi: 10.1371/journal.pgen.1002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11(6):446–450. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr LA, Kelly SE. The Genetic Knowledge Index: developing a standard measure of genetic knowledge. Genet Test. 1999;3(2):193–199. doi: 10.1089/gte.1999.3.193. [DOI] [PubMed] [Google Scholar]
- Gollust SE, Gordon ES, Zayac C, Griffin G, Christman MF, Pyeritz RE, … Bernhardt BA. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15(1):22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. “It’s not like judgment day”: public understanding of and reactions to personalized genomic risk information. J Genet Couns. 2012;21(3):423–432. doi: 10.1007/s10897-011-9476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB. Delivering pharmacogenetic testing to the masses: an achievable goal? Pharmacogenomics. 2014;15(1):1–4. doi: 10.2217/pgs.13.211. [DOI] [PubMed] [Google Scholar]
- Haga SB, Barry WT, Mills R, Ginsburg GS, Svetkey L, Sullivan J, Willard HF. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomarkers. 2013;17(4):327–335. doi: 10.1089/gtmb.2012.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, Barry WT, Mills R, Svetkey L, Suchindran S, Willard HF, Ginsburg GS. Impact of delivery models on understanding genomic risk for type 2 diabetes. Public Health Genomics. 2014;17(2):95–104. doi: 10.1159/000358413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukkala A, Konttinen H, Hankonen N, Perola M, Kaariainen H, Salomaa V. Genetic causal beliefs about morbidity: associations with health behaviors and health outcome beliefs about behavior changes between 1982–2002 in the Finnish population. BMC Public Health. 2015;15:389. doi: 10.1186/s12889-015-1657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10(1):19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- Inglis A, Koehn D, McGillivray B, Stewart SE, Austin J. Evaluating a unique, specialist psychiatric genetic counseling clinic: uptake and impact. Clin Genet. 2015;87(3):218–224. doi: 10.1111/cge.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallinoja P, Aro AR. Knowledge about genes and heredity among Finns. New Genetics and Society. 1999;18(1):101–110. [Google Scholar]
- Jenkins J, Calzone KA, Dimond E, Liewehr DJ, Steinberg SM, Jourkiv O, … Kirsch IR. Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med. 2007;9(8):487–495. doi: 10.1097/gim.0b013e31812e6220. doi:10.1097GIM.0b013e31812e6220. [DOI] [PubMed] [Google Scholar]
- Julian-Reynier C, Mancini J, Mouret-Fourme E, Gauthier-Villars M, Bonadona V, Berthet P, … Nogues C. Cancer risk management strategies and perceptions of unaffected women 5 years after predictive genetic testing for BRCA1/2 mutations. Eur J Hum Genet. 2011;19(5):500–506. doi: 10.1038/ejhg.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst KA, McBride CM, Wade C, Alford SH, Reid R, Larson E, … Brody LC. Patients’ understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;14(7):681–687. doi: 10.1038/gim.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparian NA, Wakefield CE, Meiser B. Assessment of psychosocial outcomes in genetic counseling research: an overview of available measurement scales. J Genet Couns. 2007;16(6):693–712. doi: 10.1007/s10897-007-9111-6. [DOI] [PubMed] [Google Scholar]
- Kaufman DJ, Bollinger JM, Dvoskin RL, Scott JA. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012;21(3):413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- Keller M, GE, Stack CB, et al. The Coriell Personalized Medicine Collaborative: a prospective study of the utility of personalized medicine. Personalized Med. 2010;7:301–317. doi: 10.2217/pme.10.13. [DOI] [PubMed] [Google Scholar]
- Lautenbach DM, Christensen KD, Sparks JA, Green RC. Communicating genetic risk information for common disorders in the era of genomic medicine. Annu Rev Genomics Hum Genet. 2013;14:491–513. doi: 10.1146/annurev-genom-092010-110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Hooker GW, Connors PD, Hyams TC, Wright MF, Caldwell S, … Biesecker BB. Participant use and communication of findings from exome sequencing: a mixed-methods study. Genet Med. 2015 doi: 10.1038/gim.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006;62(6):1360–1368. doi: 10.1016/j.socscimed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- McBride CM, Alford SH, Reid RJ, Larson EB, Baxevanis AD, Brody LC. Characteristics of users of online personalized genomic risk assessments: implications for physician-patient interactions. Genet Med. 2009;11(8):582–587. doi: 10.1097/GIM.0b013e3181b22c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Birmingham WC, Kinney AY. Health psychology and translational genomic research: bringing innovation to cancer-related behavioral interventions. Am Psychol. 2015;70(2):91–104. doi: 10.1037/a0036568. [DOI] [PubMed] [Google Scholar]
- McInerney-Leo A, Hadley D, Kase RG, Giambarresi TR, Struewing JP, Biesecker BB. BRCA1/2 testing in hereditary breast and ovarian cancer families III: risk perception and screening. Am J Med Genet A. 2006;140(20):2198–2206. doi: 10.1002/ajmg.a.31432. [DOI] [PubMed] [Google Scholar]
- Middleton A, Hall G, Patch C. Genetic counselors and Genomic Counseling in the United Kingdom. Mol Genet Genomic Med. 2015;3(2):79–83. doi: 10.1002/mgg3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R, Haga SB. Genomic counseling: next generation counseling. J Genet Couns. 2014;23(4):689–692. doi: 10.1007/s10897-013-9641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RH, Kiely DK, Cupples LA, Kannel WB. Parental history is an independent risk factor for coronary artery disease: the Framingham Study. Am Heart J. 1990;120(4):963–969. doi: 10.1016/0002-8703(90)90216-k. [DOI] [PubMed] [Google Scholar]
- National Society of Genetic Counselors’ Definition Task F. Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, … Williams JL. A new definition of Genetic Counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- O’Daniel JM. The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. J Genet Couns. 2010;19(4):315–327. doi: 10.1007/s10897-010-9302-4. [DOI] [PubMed] [Google Scholar]
- O’Neill SC, McBride CM, Alford SH, Kaphingst KA. Preferences for genetic and behavioral health information: the impact of risk factors and disease attributions. Ann Behav Med. 2010;40(2):127–137. doi: 10.1007/s12160-010-9197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond KE. From genetic counseling to “genomic counseling”. Mol Genet Genomic Med. 2013;1(4):189–193. doi: 10.1002/mgg3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne K, Nicholls S, McAllister M, Macleod R, Donnai D, Davies LM. Outcome measurement in clinical genetics services: a systematic review of validated measures. Value Health. 2008;11(3):497–508. doi: 10.1111/j.1524-4733.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- Roberts JS, Dolinoy DC, Tarini BA. Emerging issues in public health genomics. Annu Rev Genomics Hum Genet. 2014;15:461–480. doi: 10.1146/annurev-genom-090413-025514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson SC, O’Neill SC, White DB, Bepler G, Bastian L, Lipkus IM, McBride CM. Responses to online GSTM1 genetic test results among smokers related to patients with lung cancer: a pilot study. Cancer Epidemiol Biomarkers Prev. 2009;18(7):1953–1961. doi: 10.1158/1055-9965.EPI-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlen T, Gordon ES, Christman MF. Genomic Literacy: Emerging Themes Among Genetic Counseling Inquiries From Participants of the Coriell Personalized Medicine Collaborative. Paper presented at the From 28th Annual Education Conference of the National Society of Genetic Counselors; Atlanta, Georgia. 2009. [Google Scholar]
- Schmidlen T, SL, Zhaoyang R, Kasper R, Sweet K, Gordon ES, Keller M, Stack C, Gharani N, Daly MB, Jarvis J, Christman M. Genetic Knowledge Among Participants in the Coriell Personalized Medicine Collaborative. J Genet Couns. 2016;2:385–394. doi: 10.1007/s10897-015-9883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlen T, Wawak L, Kasper R, Garcia-Espana JF, Christman MF, Gordon ES. Personalized genomic results: analysis of informational needs. J Genet Couns. 2014;23(4):578–587. doi: 10.1007/s10897-014-9693-8. [DOI] [PubMed] [Google Scholar]
- Schmidlen TJ, Scheinfeldt L, Zhaoyang R, Kasper R, Sweet K, Gordon ES, … Christman MF. Genetic Knowledge Among Participants in the Coriell Personalized Medicine Collaborative. J Genet Couns. 2016;25(2):385–394. doi: 10.1007/s10897-015-9883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton CA, Whitcomb DC. Evolving Roles for Physicians and Genetic Counselors in Managing Complex Genetic Disorders. Clin Transl Gastroenterol. 2015;6:e124. doi: 10.1038/ctg.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh S, deHeer HD, Peleg S, Hensley Alford S, Skapinsky K, Roberts JS, Hadley DW. The impact of multiplex genetic testing on disease risk perceptions. Clin Genet. 2015;87(2):117–123. doi: 10.1111/cge.12403. [DOI] [PubMed] [Google Scholar]
- Smerecnik CM, Mesters I, Verweij E, de Vries NK, de Vries H. A systematic review of the impact of genetic counseling on risk perception accuracy. J Genet Couns. 2009;18(3):217–228. doi: 10.1007/s10897-008-9210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet K, Gordon ES, Sturm AC, Schmidlen TJ, Manickam K, Toland AE, … Marsh C. Design and implementation of a randomized controlled trial of genomic counseling for patients with chronic disease. J Pers Med. 2014;4(1):1–19. doi: 10.3390/jpm4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet K, Sturm AC, Rettig A, McElroy J, Agnese D. Clinically relevant lessons from Family HealthLink: a cancer and coronary heart disease familial risk assessment tool. Genet Med. 2015;17(6):493–500. doi: 10.1038/gim.2014.136. [DOI] [PubMed] [Google Scholar]
- Sweet K, Sturm AC, Schmidlen T, Hovick S, Peng J, Manickam K, … Christman M. EMR documentation of physician-patient communication following genomic counseling for actionable complex disease and pharmacogenomic results. Clin Genet. 2016 doi: 10.1111/cge.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier AM, Allain DC. Models of service delivery for cancer genetic risk assessment and counseling. J Genet Couns. 2014;23(2):239–253. doi: 10.1007/s10897-013-9655-6. [DOI] [PubMed] [Google Scholar]
- Vassy JL, O’Brien KE, Waxler JL, Park ER, Delahanty LM, Florez JC, … Grant RW. Impact of literacy and numeracy on motivation for behavior change after diabetes genetic risk testing. Med Decis Making. 2012;32(4):606–615. doi: 10.1177/0272989X11431608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veach PM, Bartels DM, Leroy BS. Coming full circle: a reciprocal-engagement model of genetic counseling practice. J Genet Couns. 2007;16(6):713–728. doi: 10.1007/s10897-007-9113-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Reported Disease Risk Values for a Caucasian OSU-CPMC Participant
Table SII. Self-Reported Diagnoses of Study Participants
Table SIII. Number of Participants with at Least One Risk Factor
Table SIV. Number of Days Between Survey Completion, Report Release, Report Viewing and Genomic Counseling
Table SV. Causal Attributes of Genetic Variant, Family History, and Environmental Disease Risk: General Composite Score Across All Eight Diseases Under Study (Intention to Treat)
Table SVI. Baseline Personal Awareness of Family History and Environmental Risk Factors
Table SVII. Participant Awareness of an Increase in Disease Risk Due to a Genetic Variant (Intention to Treat)
Table SVIII. Perceived Risk (Intention to Treat)
Table SIX. Response to Numeracy Questions at Baseline and Follow up
Table SX. General and Relative Risk Numeracy, Combined Question Response
Table SXI. Personal Rating of Genetic Knowledge
Table SXII. Complex and Simple Genetic/Genomic Knowledge Questions
Table SXIII. Follow up (Complex/Simple) Genetic/Genomic Knowledge Adjusted for Covariates (including Numeracy)
Table SXIV. Confidence in Use of Results, Genomic Counseling Satisfaction