Scheme 4.
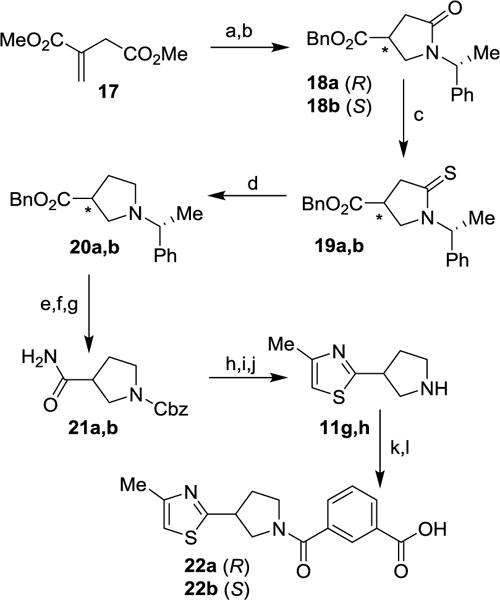
Reagents and conditions: (a) (R)-α-methylbenzylamine MeOH, 115 °C, 18 h, 75%; (b) BnOH, PPTS, toluene, reflux, 24 h, (S) 81%, (R) 73%; (c) Lawesson’s reagent, toluene, 100 °C, 2 h, (S) 99%, (R) 67%; (d) MeI, CH2Cl2, 25 °C, 24 h then NaBH4, EtOH, 0 °C, 1h, (S) 50%, (R) 38%; (e) H2, 10% Pd/C, 25 °C, 20 h, (S) 99%, (R) 99%; (f) CbzCl, 4 N NaOH, 0 °C, 2 h, (S) 51%, (R) 39%; (g) Boc2O, pyridine, NH4HCO3, 1,4-dioxane, 25 °C, 16 h; (h) Lawesson’s reagent, THF, 25 °C, 16 h; (i) chloroacetone, EtOH, 80 °C, 18 h, (S) 42%, (R) 56% (over 3 steps); (j) HBr in AcOH, 25 °C, 1 h, (S) 89%, (R) 88%; (k) 7a, EDCI, HOBt, i-Pr2NEt, CH2Cl2, 25 °C, 16 h, (S) 55%, (R) 68%; (l) LiOH.H2O, THF/MeOH/H2O (3:1:1), 25 °C, 16 h, (S) 55%, (R) 54%.
