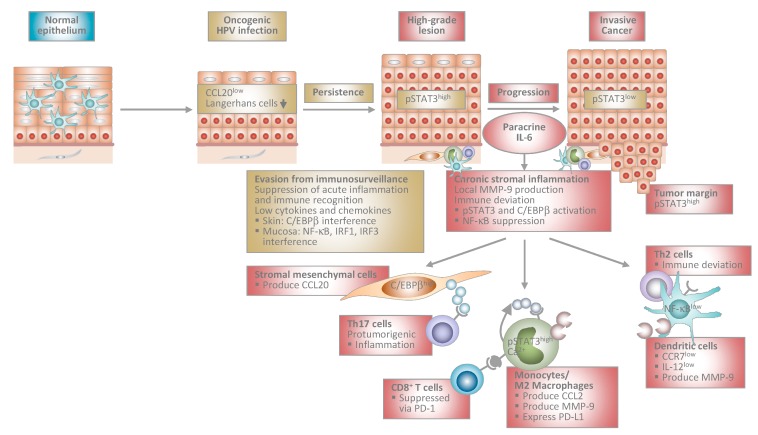
Figure 1.
Proposed model of human papillomavirus-induced carcinogenesis. Stage-specific interplay between virally infected keratinocytes and the local immune microenvironment. At early stages, HPV-infected cells suppress acute inflammation in the epithelium and immune recognition. This allows escape from immunosurveillance and viral persistence. During progression to invasive cancer HPV-transformed cells initiate chronic stromal inflammation and immune deviation orchestrated by paracrine IL-6. The IL-6/STAT3 and IL-6/C/EBPβ pathways lead to chemokine induction in stromal mesenchymal and infiltrating immune cells. As a consequence, myelomonocytic cells expressing protumorigenic MMP-9 and Th17 cells are recruited further promoting inflammation. Myelomonocytic cells differentiate into functionally impaired dendritic cells or M2 macrophages expressing PD-L1 that inhibit cytotoxic T cell responses. IL-6 suppresses NF-κB activity in stromal dendritic cells, which are unable to migrate in response to lymph node homing chemokines due to low CCR7 chemokine receptor expression. Instead, they are immobilized within the tumor stroma and produce MMP-9 locally. IL-12 is expressed only at low levels shifting T helper cell responses from Th1 to Th2. Stromal inflammation and immune deviation facilitate progression to invasiveness. HPV: human papillomavirus; IL: interleukin; STAT3: signal transducer and activator of transcription 3; C/EBP: CCAAT/enhancer binding protein; MMP: matrix-metalloproteinase; Th: T helper; PD-L1: programmed death-ligand 1; NF: nuclear factor; CCR: C-C chemokine receptor; CCL: C-C chemokine ligand; IRF: interferon regulatory factor.