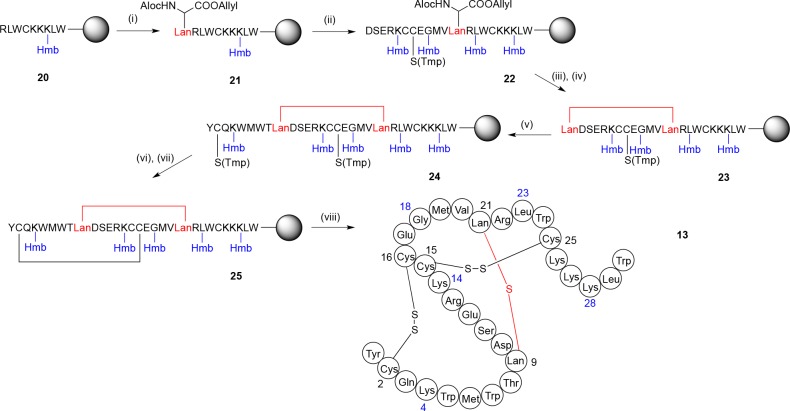
Scheme 3. Synthesis of Lanthionine-Bridged ProTx-II Analogue 13.
Standard protecting groups were used for the amino acids, with additional Hmb protection as indicated; see Experimental Section. Reagents and conditions: (i) 1, PyAOP, HOAt, DIPEA, DMF, μwave, 5 min, 60 °C; (ii) incorporation of standard protected amino acids with HBTU, DIPEA, DMF, followed by deprotection with piperidine; (iii) 40% piperidine/DMF, then Pd(PPh3)4, 1,3-dimethylbarbituric acid, DMF, CH2Cl2; (iv) PyAOP, HOAt, DIPEA, DMF, μwave, 5 min, 60 °C; (v) incorporation of standard protected amino acids with HBTU, DIPEA, DMF, followed by deprotection with piperidine; (vi) 5% DTT in 0.1 M NMM, DMF; (vii) NCS (2 equiv), DMF; (viii) TFA, iPr3SiH, H2O.