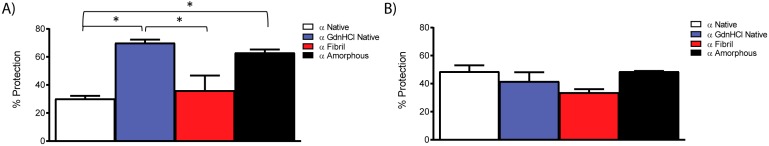
Figure 3.
Chaperone protection provided by native, fibrillar and amorphous α-crystallin species against the (A) amorphous aggregation of reduced insulin, 250 μg/mL in 0.1 M sodium phosphate, pH 7.4 and 20 mM DTT at 37 °C (0.9 chaperone: 1.0 insulin on a molar basis); and (B) fibrillar aggregation of RCM-κ-casein, 400 μg/mL incubated at 37 °C for 22 h in the presence of various native, amorphous and fibrillar chaperone species (0.5 chaperone: 1.0 RCM κ-casein on a molar basis) and monitored via ThT fluorescence. The percentage of protection provided by each chaperone is calculated from the difference between the maximal light scattering or fluorescence of the target protein alone and the target protein in the presence of the stated concentrations of α-crystallin. Results are mean ± SE of the percentage protection given by chaperones for three experiments; p-values, derived by one-way ANOVA with Tukey post-test, are * p < 0.05.