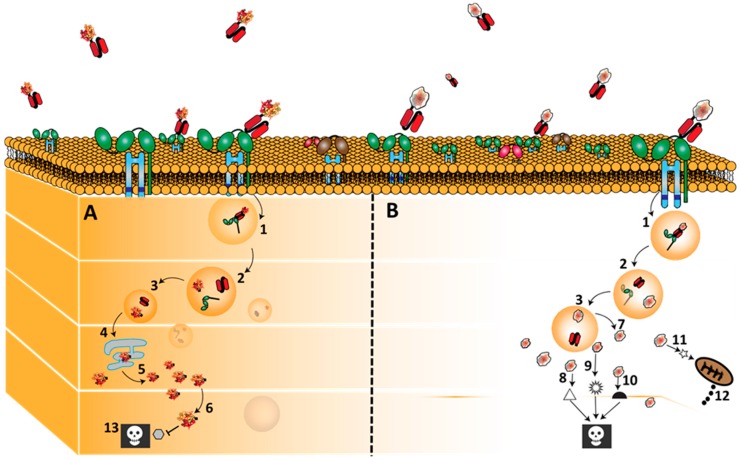
Figure 3.
Binding, internalization and routing of CD64 directed bacteria immunotoxins (ETA’) and human cytolytic fusion protein (Granzyme B). H22 based fusion proteins bind CD64 and are internalize by receptor mediated endocytosis after which they are processed in the endosomal compartment. As mentioned earlier, the targeted killing of M1 macrophages by these fusion proteins is due to the differential post-internalization kinetics of these fusion proteins in M1 macrophages versus endosomal degradation in the M2-type macrophages. Once, internalized, a furin cleavage site separates the ligand from the effector molecules (1–3). ETA’ (Panel A) naturally contains a translocation domain by which it leaves the endosome and traffics through the endoplasmic reticulum into the cytosol (4–6) Here it enzymatically inactivates protein synthesis by ADP-ribosylation of EF-2,which subsequently leads to cell death (13). On the other hand, granzyme B based fusion proteins (Panel B) are often engineered to contain an adapter domain that allows their translocation into the cytosol (7) where they initiate apoptosis by cleaving; caspase dependent substrates (8), caspase independent substrates (9), BH3-only pro-apoptotic protein (Bid) (11) with subsequent cytochrome C release (12). Note (10): Some other intracellular apoptosis inducing pathway not yet identified.