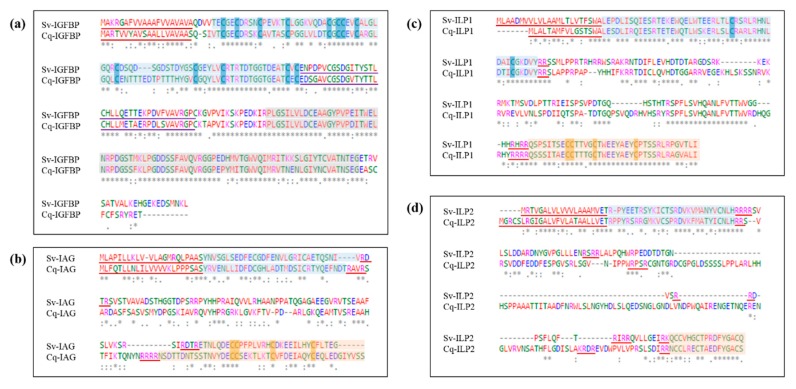
Figure 2.
Sequence alignment of Sagmariasus verreauxi and Cherax quadricarinatus IGFBP and ILP homologues, with emphasis on the conservation of physicochemical properties. Residues are colored in accordance with properties: red—defines small, hydrophobic residues; blue—negatively charged/acidic; magenta—positively charged/basic; and green—polar and amine groups. An asterisk (*) indicates a conserved amino acid, a colon (:) those with conserved physicochemical properties, and a full stop (.) those with weakly similar properties. (a) Compares Cq-IGFBP and Sv-IGFBP, the signal peptide is underlined in red, the insulin-binding domain boxed in blue with the cysteine core highlighted, the kazal domain underlined in purple, and the C’ immunoglobulin domain boxed in grey. (b) Compares Cq-IAG and Sv-IAG; (c) Sv-ILP1 and the novel Cq-ILP1; and (d) the novel Sv-ILP2 and Cq-ILP2. In each case the signal peptide is underlined in red and the mature hormone is highlighted as the B-(blue) and A-(orange) chains with the cysteine core of each highlighted; C-peptide Arg-C proteinase cleavage sites are underlined in red.