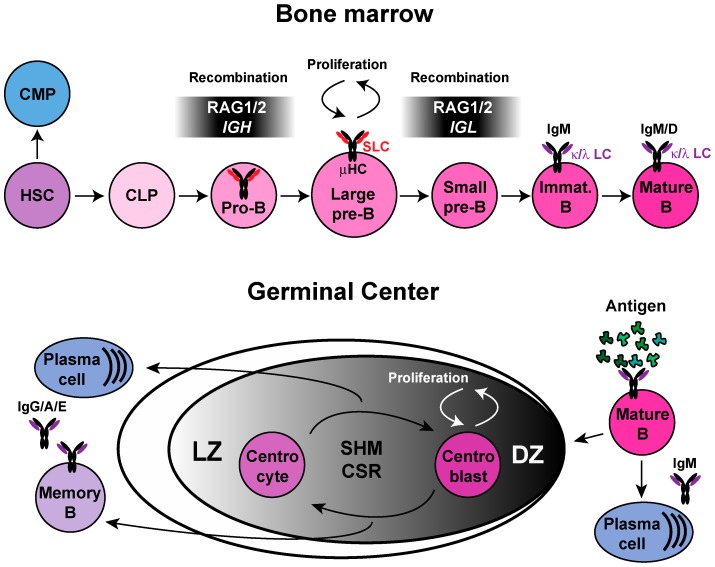
Figure 1.
Antigen-independent B-cell development in the bone marrow, and antigen-dependent B-cell activation and differentiation in the germinal center. B cells develop in an antigen-independent fashion from common lymphocyte progenitors (CLP) in the bone marrow. The recombination activating gene 1/2 complex (RAG1/2) is expressed in pro-B cells, which, when leading to successful immunoglobulin (Ig) heavy chain recombination and expression, results in the formation of the pre-B cell receptor (pre-BCR) upon pairing with the surrogate light chain (SLC). Clonal expansion takes place in large pre-B cells, where RAG1/2 expression is downregulated. Pre-BCR signaling is involved in cell-cycle exit, re-expression of RAG1/2, and Ig light chain recombination. Upon expression of a complete IgM molecule, B cells fully mature and exit the bone marrow. Antigen-dependent B-cell activation in secondary lymphoid organs initiates the germinal center reaction, where antigen-specific B cells undergo affinity maturation through iterative rounds of Ig gene somatic hypermutations (SHM), and undergo Ig class switch recombination (CSR). Antigen-dependent selection leads to memory B-cell and plasma cell differentiation.