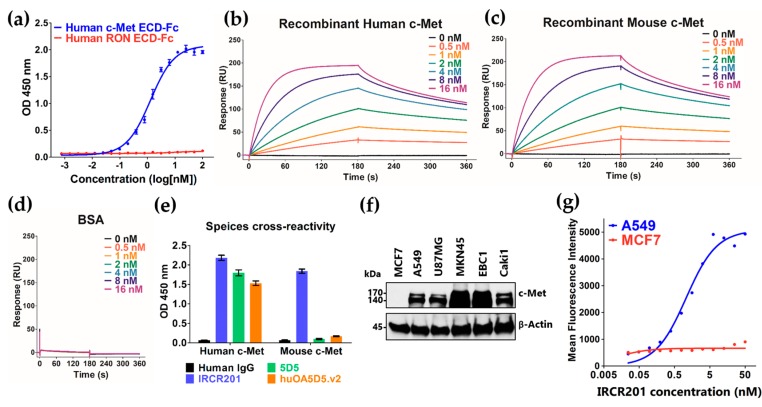
Figure 1.
Binding properties of IRCR201. (a) Binding patterns of IRCR201 to the human c-Met extracellular domain (ECD)-fragment crystallizable region (Fc) and the human RON (recepteur d’origine nantais) ECD-Fc were measured by enzyme-linked immunosorbent assay (ELISA). IRCR201 binds to the human c-Met ECD-Fc with specificity and selectivity; (b–d) Surface plasmon resonance (SPR) sensorgrams binding with varying concentrations of IRCR201 to human c-Met, mouse c-Met, or bovine serum albumin (BSA) immobilized onto a CM5 BiacoreTM sensor chip; (e) Cross-reactivity analysis of IRCR201 to human and mouse c-Met; (f) Analysis of c-Met expression in various types of cancer cell lines; (g) Binding analysis of IRCR201 to the cell surface c-Met through a flow cytometer (FACSAria™ III).