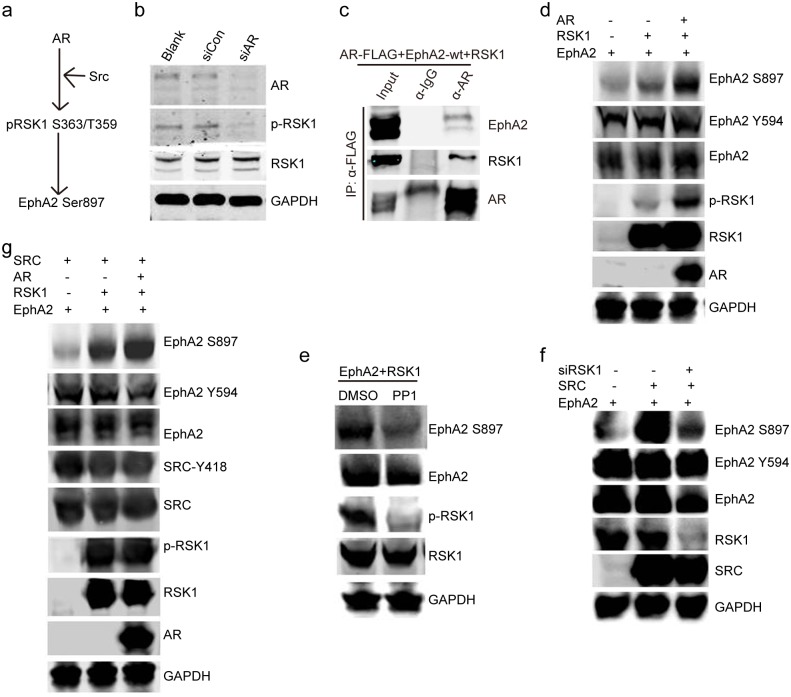
Fig 7. AR activates RSK1, the kinase that phosphorylates EphA2 at Ser897, by recruiting Src.
(a) Schematic demonstration of AR-mediated upregulation of the phosphorylation of EphA2 at Ser897. (b) AR inhibition leads to reduced activation of RSK1. siRNA-transfected cells were subjected to western blotting. (c) The interaction between AR and RSK1 was verified. Plasmids expressing FLAG-tagged AR and RSK1 were transfected into 293T cells. 48 h later, the cells were subjected to immunoprecipitation using anti-FLAG M2 affinity beads, followed by western blotting. (d) Ectopic AR further promotes the expression of pEphA2 Ser897 upregulated by RSK1. Expression plasmids were transfected into basic medium-cultured 293T cells in the indicated combinations. (e) Expression of phosphorylated RSK1 is considerably impaired by the Src inhibitor PP1. 293T cells were cultured, and then co-transfected with plasmids expressing GFP-tagged EphA2 and hemagglutinin-tagged RSK1 for 48 h prior to the application of PP1 (at 5μM) for 4 h. (f) RSK1 is required by Src to activate the pEphA2 Ser897. siRNA targeting RSK1 and the control siRNAs were transfected into basic medium-cultured 293T cells for 24h, followed by transfection of expression plasmids in indicated combinations. After 36 h, cells were harvested. (g) Src andRSK1 upregulate the phosphorylation of EphA2 at Ser897, and the effect is mediated by the AR. Expression plasmids were transfected into basic medium-cultured 293T cells in the indicated combinations. Each reaction was repeated in, at least, triplicate.