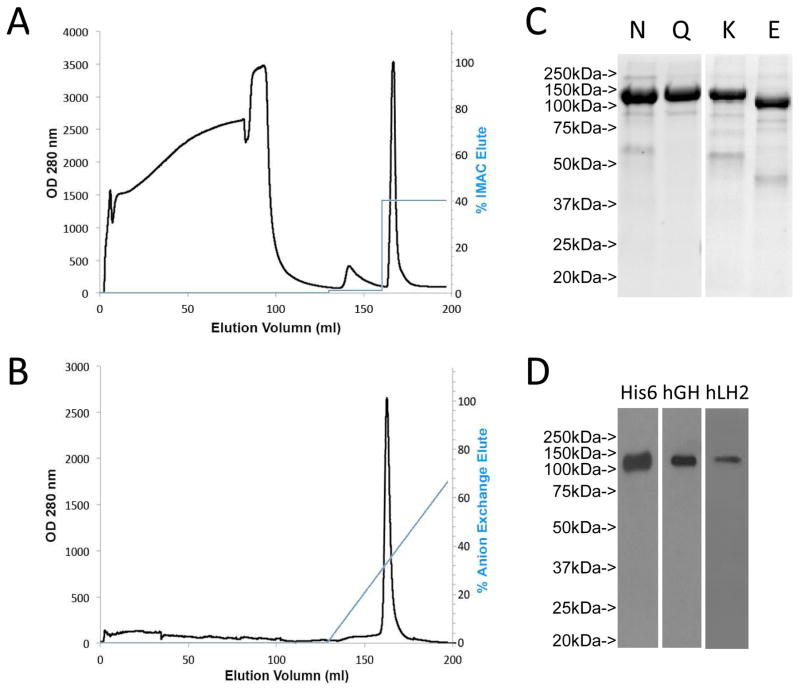
Figure 1.
Two step LH2 protein purification (A) LH2 was purified by immobilized metal ion affinity chromatograph (IMAC) of concentrated, buffer-exchanged conditioned medium samples taken from CHO cells transiently transfected with a vector expressing human LH2 tagged with His8 and human growth hormone (hGH). The protein was eluted with 400 mM imidazole as a single peak. (B) Anion exchange chromatography purification of LH2 recombinant protein. (C) SDS-polyacrylamide gel electrophoresis of LH2 protein after IMAC (N) and anion exchange chromatography (Q) purification. LH2 protein isolated from Kifunesine treated cells (K) showed a narrower band, which shifted down by ~25 kDa after EndoH treatment (E), suggesting LH2 is glycosylated. The positions and sizes of molecular weight markers (MW) are indicated. (D) The identity of LH2 protein was confirmed by Western blotting with anti-His6, anti-hGH and anti-human LH2 antibodies.