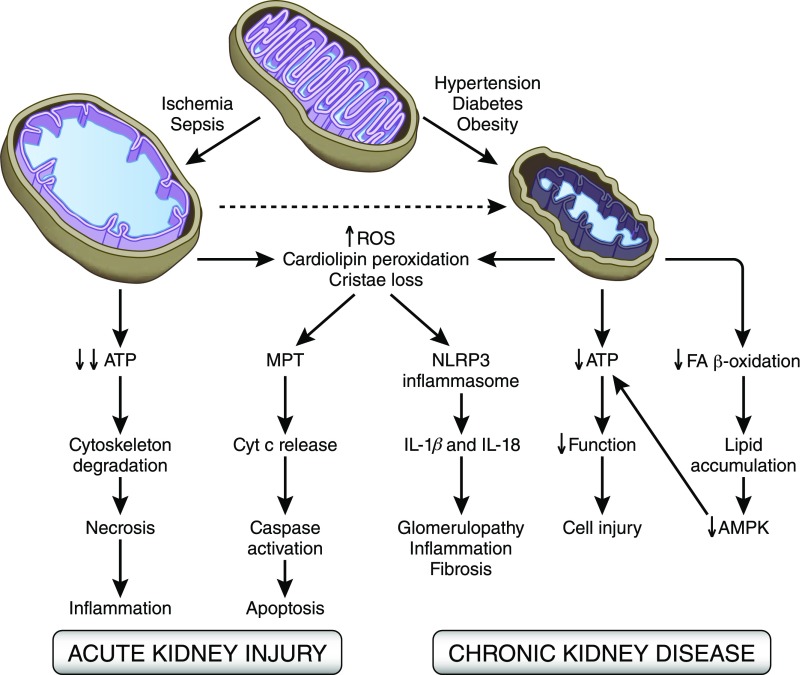
Figure 4.
Mitochondrial changes in animal models of AKI and CKD. Acute stressors, such as ischemia, sepsis, and toxins, cause mitochondrial matrix swelling and loss of cristae membrane. These structural changes are associated with impaired ATP production, oxidative stress, cellular demise, and chronic tissue remodeling. Cardiolipin peroxidation sets the stage for mitochondrial permeability transition, cytochrome c release, and apoptosis. In CKD, mitochondria are small, with reduced matrix density and loss of cristae membranes. Mitochondrial dysfunction causes a drop in ATP levels and cell injury, whereas inhibition of FA oxidation results in cellular lipid accumulation and downregulation of AMPK activity, further contributing to bioenergetics failure. In both AKI and CKD, cardiolipin peroxidation causes the translocation of cardiolipin to the OMM, where it serves as a docking station for NLRP3 inflammasome assembly. Mitochondrial ROS then triggers inflammasome activation to produce IL-1β and IL-18 that sustain tissue remodeling. MPT, mitochondrial permeabiltiy transition; NLRPE, NLR family pyrin domain containing 3.