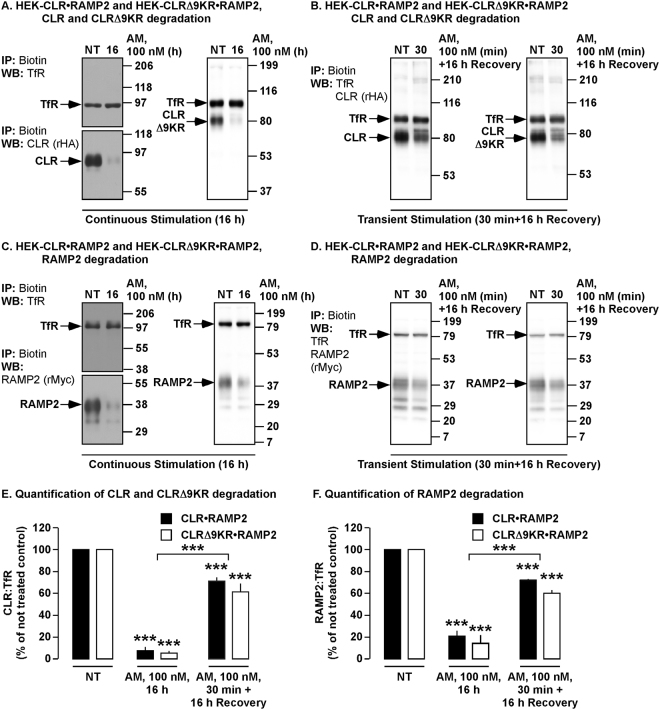
Figure 4.
AM induces degradation of CLR, CLRΔ9KR and RAMP2 following continuous and transient challenges. Cell-surface biotinylated HEK-CLR•RAMP2 and HEK-CLRΔ9KR•RAMP2 cells were not treated (NT) or challenged with AM (100 nM, as indicated), biotinylated proteins immunoprecipitated (IP) and Western blots (WB) probed for CLR (rabbit-HA, rHA), CLRΔ9KR (rHA), RAMP2 (rMyc) and transferrin receptor (TfR, loading control). (A,B) In untreated HEK-CLR•RAMP2 and HEK-CLRΔ9KR•RAMP2 cells, CLR, CLRΔ9KR, RAMP2 and TfR were readily detected. AM (100 nM, 16 h) induced degradation of CLR, CLRΔ9KR and RAMP2 to similar levels. (C,D) In untreated HEK-CLR•RAMP2 and HEK-CLRΔ9KR•RAMP2 cells, CLR, CLRΔ9KR, RAMP2 and TfR were readily detected. AM (100 nM, 30 min, followed by 16 h incubation on AM-free medium) induced degradation of CLR, CLRΔ9KR and RAMP2 to similar levels. (E,F) Quantification of the degradation of CLR and CLRΔ9KR and RAMP2. n = 3–4. Data were compared by Student’s t test and differences among multiple groups were examined using ANOVA and Student-Newman-Keuls post-hoc test, ***p < 0.001. Full length blots of panels A and C are shown in Supplementary Fig. 4.