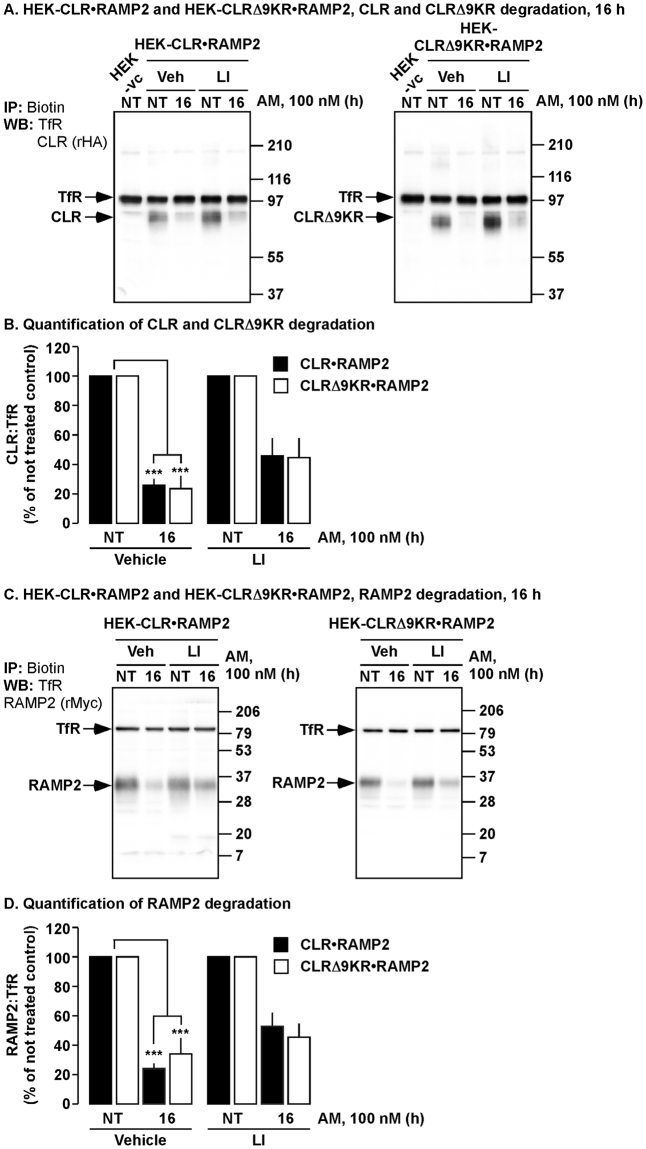
Figure 5.
Inhibition of lysosomal peptidases partially prevents degradation of CLR•RAMP2 and CLRΔ9KR•RAMP2. (A, C) Cell-surface biotinylated HEK-CLR•RAMP2 and HEK-CLRΔ9KR•RAMP2 cells were incubated with vehicle (control), lysosomal peptidase inhibitors (LI), not treated (NT) or challenged with AM (100 nM, 16 h), biotinylated proteins immunoprecipitated (IP) and Western blots (WB) probed for CLR (rabbit-HA, rHA), CLRΔ9KR (rHA), RAMP2 (rabbit-Myc, rMyc) and transferrin receptor (TfR, loading control). In untreated HEK-CLR•RAMP2 and HEK-CLRΔ9KR•RAMP2 cells, CLR, CLRΔ9KR, RAMP2 and TfR were readily detected. AM (100 nM, 16 h) induced degradation of CLR, CLRΔ9KR and RAMP2 to similar levels. (B) Quantification of the degradation of CLR and CLRΔ9KR, respectively. (D) Quantification of the degradation of RAMP2. n = 4, Data were compared by Student’s t test, *p < 0.05, HEK-vc = HEK-vector control.