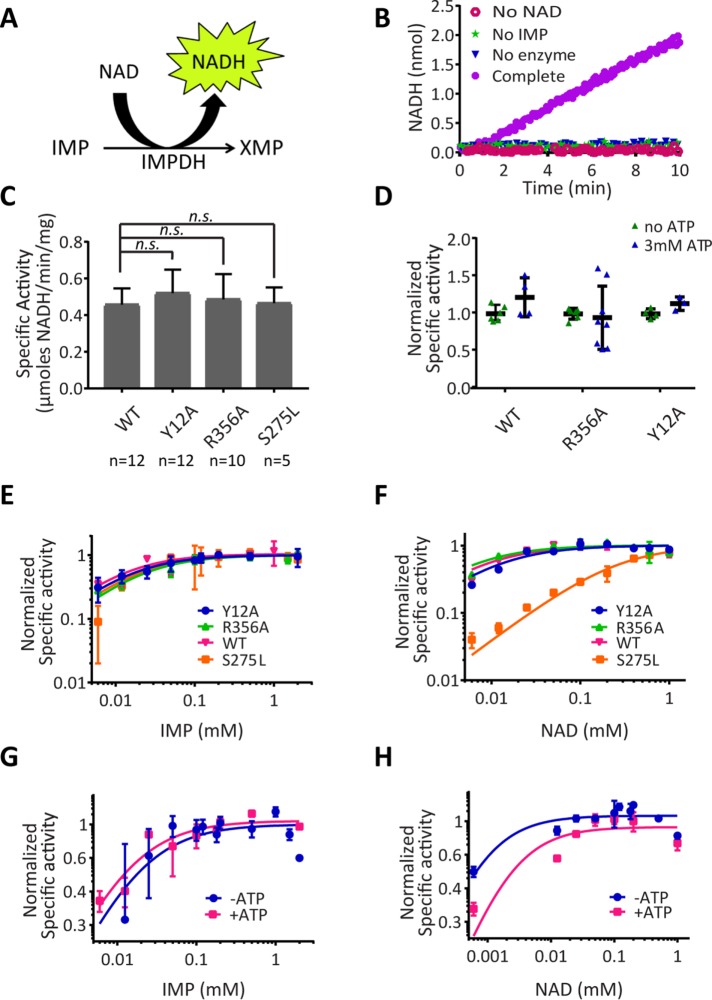
FIGURE 2:
IMPDH2 polymerization does not alter its catalytic activity in vitro. (A) Schematic of the IMPDH reaction. (B) NADH fluorescence was monitored in reactions lacking NAD+, IMP, IMPDH2 (no enzyme), or a reaction containing all components (Complete). (C) Wild-type and mutant IMPDH2 activity under nonassembling conditions. All mutants showed no significant difference from wild type (two-sided Student’s t test; p > 0.05). Bars denote mean and SD. (D) IMPDH activity assays as in C were conducted in the absence or presence of ATP to induce assembly. ATP did not significantly alter activity in any case (two-sided Student’s t test). (E, F) Initial reaction rates are plotted as a function of substrate concentration for IMP or NAD+ comparing wild-type and mutant IMPDH2 under polymerization conditions (1 mM ATP) or for wild type in the presence or absence of ATP (G, H). Michaelis constants determined from these titrations are given in Supplemental Table 1. Two to five replicates per substrate concentration.