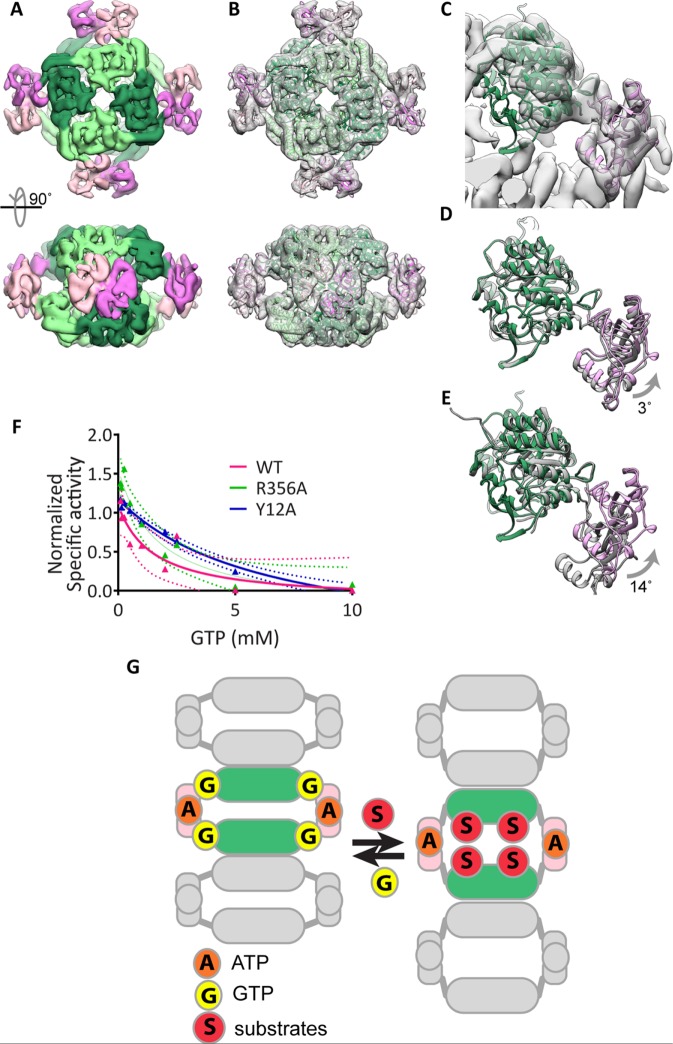
FIGURE 5:
GTP-bound human IMPDH2 adopts the collapsed, inhibited conformation. (A) Cryo-EM reconstruction of Y12A in the presence of 0.1 mM ATP and GTP at 8.7 Å resolution. Catalytic (greens) and Bateman (pinks) domains are well resolved. (B) Atomic model of IMPDH2-ATP-GTP, generated by fitting the catalytic domain and Bateman domains into the cryo-EM structure as two separate rigid bodies. (C) Close-up view of a single IMPDH monomer fitted in the cryo-EM structure. (D) A monomer of the A. gossypii IMPDH-GDP crystal structure (gray) (PDB ID 4Z87) aligned to the human IMPDH2-ATP-GTP model (color) or to the P. aueriginosa IMPDH-ATP monomer (E). (F) Dose-dependent inhibition of wild-type and mutant IMPDH2 by GTP. IMPDH2 was incubated with 1 mM ATP and 3 mM IMP to promote polymerization and then GTP was added for 10 min prior to reaction initiation by with 5 mM NAD+. Mean values are plotted and fitted to a three-parameter dose–response curve. Each data point represents three to six replicate reactions. Ninety-five-percent confidence intervals are plotted as dotted lines of the corresponding color. (G) Model: human IMPDH exists in a conformational equilibrium between an expanded, active conformation and a collapsed, inactive conformation that can be shifted by binding to substrates or GTP. Unlike other metabolic filaments, the conformational equilibrium of IMPDH is unaffected by polymerization because the filament form can accommodate both active and inactive conformations.